Functional Dissection of Genes Encoding DNA Polymerases Based on Conditional Mutants in the Heterocyst-Forming Cyanobacterium Anabaena PCC 7120
- PMID: 32582078
- PMCID: PMC7283527
- DOI: 10.3389/fmicb.2020.01108
Functional Dissection of Genes Encoding DNA Polymerases Based on Conditional Mutants in the Heterocyst-Forming Cyanobacterium Anabaena PCC 7120
Abstract
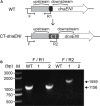
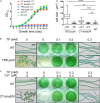
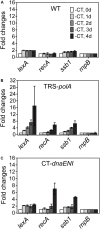
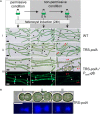
The filamentous cyanobacterium Anabaena sp. PCC 7120 develops N2-fixing heterocyst cells under condition of combined-nitrogen deprivation and constitutes an excellent model for studying cell differentiation. The mechanism of heterocyst development has been extensively investigated and a network of regulating factors has been identified. A few studies have showed that the process of heterocyst differentiation relates with cell cycle events, but further investigation is still required to understand this relationship. In a previous study, we created a conditional mutant of PolI encoding gene, polA, by using a CRISPR/Cpf1 gene-editing technique. Here, we were able to create another conditional mutant of a PolIII encoding gene dnaENI using a similar strategy and subsequently confirmed the essential roles of both polA and dnaENI in DNA replication. Further investigation on the phenotype of the mutants showed that lack of PolI caused defects in chromosome segregation and cell division, while lack of DnaENI (PolIII) prevented bulk DNA synthesis, causing significant loss of DNA content. Our findings also suggested the possible existence of a SOS-response like mechanism operating in Anabaena PCC 7120. Moreover, we found that heterocyst development was differently affected in the two conditional mutants, with double heterocysts/proheterocysts found in PolI conditional mutant. We further showed that formation of such double heterocysts/proheterocysts are likely caused by the difficulty in nucleoids segregation, resulting delayed, or non-complete closure of the septum between the two daughter cells. This study uncovers a link between DNA replication process and heterocyst differentiation, paving the way for further studies on the relationship between cell cycle and cell development.
Keywords: DNA replication; SOS response; cell cycle; cyanobacteria; heterocyst.
Copyright © 2020 Xing, Xie, Zeng, Yang and Zhang.
Figures

References
-
- Borthakur P. B., Orozco C. C., Young-Robbins S. S., Haselkorn R., Callahan S. M. (2005). Inactivation of patS and hetN causes lethal levels of heterocyst differentiation in the filamentous cyanobacterium Anabaena sp. PCC 7120. Mol. Microbiol. 57 111–123. 10.1111/j.1365-2958.2005.04678.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

