Regulation of Apoptosis by Enteroviruses
- PMID: 32582091
- PMCID: PMC7283464
- DOI: 10.3389/fmicb.2020.01145
Regulation of Apoptosis by Enteroviruses
Abstract
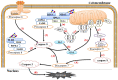
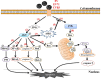
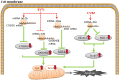
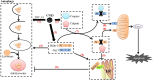
Enterovirus infection can cause a variety of diseases and severely impair the health of humans, animals, poultry, and other organisms. To resist viral infection, host organisms clear infected cells and viruses via apoptosis. However, throughout their long-term competition with host cells, enteroviruses have evolved a series of mechanisms to regulate the balance of apoptosis in order to replicate and proliferate. In the early stage of infection, enteroviruses mainly inhibit apoptosis by regulating the PI3K/Akt pathway and the autophagy pathway and by impairing cell sensors, thereby delaying viral replication. In the late stage of infection, enteroviruses mainly regulate apoptotic pathways and the host translation process via various viral proteins, ultimately inducing apoptosis. This paper discusses the means by which these two phenomena are balanced in enteroviruses to produce virus-favoring conditions - in a temporal sequence or through competition with each other. This information is important for further elucidation of the relevant mechanisms of acute infection by enteroviruses and other members of the picornavirus family.
Keywords: apoptotic pathway; balance; enterovirus; regulation; viral replication.
Copyright © 2020 Lai, Wang, Cheng, Mao, Ou, Yang, Wu, Jia, Liu, Zhu, Chen, Zhang, Zhao, Huang, Gao, Wang, Xu, Chen, Zhu, Luo, Liu, Yu, Zhang, Tian, Pan, Rehman and Chen.
Figures
References
-
- Bagchi P., Dutta D., Chattopadhyay S., Mukherjee A., Halder U. C., Sarkar S., et al. (2010). Rotavirus nonstructural protein 1 suppresses virus-induced cellular apoptosis to facilitate viral growth by activating the cell survival pathways during early stages of infection. J. Virol. 84 6834–6845. 10.1128/jvi.00225-10 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

