Adjuvanting Allergen Extracts for Sublingual Immunotherapy: Calcitriol Downregulates CXCL8 Production in Primary Sublingual Epithelial Cells
- PMID: 32582164
- PMCID: PMC7295906
- DOI: 10.3389/fimmu.2020.01033
Adjuvanting Allergen Extracts for Sublingual Immunotherapy: Calcitriol Downregulates CXCL8 Production in Primary Sublingual Epithelial Cells
Abstract
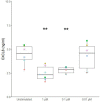
Application of allergens onto the sublingual epithelium is used to desensitize allergic individuals, a treatment known as sublingual immunotherapy. However, the response of sublingual epithelial cells to house dust mite allergen and potential tolerance-promoting adjuvants such as Toll-like receptor (TLR) ligands and calcitriol has not been investigated. In order to study this, primary sublingual epithelial cells were isolated from dogs and cultured in vitro. After 24-h incubation with a Dermatophagoides farinae extract, a Dermatophagoides pteronyssinus extract, TLR2 ligands (FSL-1, heat-killed Listeria monocytogenes, Pam3CSK4), a TLR3 ligand (poly I:C), a TLR4 ligand [lipopolysaccharide (LPS)], and calcitriol (1,25-dihydroxyvitamin D3), viability of the cells was analyzed using an MTT test, and their secretion of interleukin 6 (IL-6), IL-10, CXCL8, and transforming growth factor β1 (TGF-β1) was measured by enzyme-linked immunosorbent assay. Additionally, to evaluate its potential effect as an adjuvant, sublingual epithelial cells were incubated with calcitriol in combination with a D. farinae extract followed by measurement of CXCL8 secretion. Furthermore, the effect of D. farinae and calcitriol on the transcriptome was assessed by RNA sequencing. The viability of the sublingual epithelial cells was significantly decreased by poly I:C, but not by the other stimuli. CXCL8 secretion was significantly increased by D. farinae extract and all TLR ligands apart from LPS. Calcitriol significantly decreased CXCL8 secretion, and coadministration with D. farinae extract reduced CXCL8 concentrations to levels seen in unstimulated sublingual epithelial cells. Although detectable, TGF-β1 secretion could not be modulated by any of the stimuli. Interleukin 6 and IL-10 could not be detected at the protein or at the mRNA level. It can be concluded that a D. farinae extract and TLR ligands augment the secretion of the proinflammatory chemokine CXCL8, which might interfere with sublingual desensitization. On the other hand, CXCL8 secretion was reduced by coapplication of calcitriol and a D. farinae extract. Calcitriol therefore seems to be a suitable candidate to be used as adjuvant during sublingual immunotherapy.
Keywords: CXCL8; Dermatophagoides farinae; Toll-like receptor; calcitriol; dog; epithelium; sublingual; sublingual immunotherapy.
Copyright © 2020 Pelst, Höbart, Wallaeys, De Rooster, Gansemans, Van Nieuwerburgh, Devriendt and Cox.
Figures





References
-
- Maina E, Cox E. A double blind, randomized, placebo controlled trial of the efficacy, quality of life and safety of food allergen-specific sublingual immunotherapy in client owned dogs with adverse food reactions: a small pilot study. Vet Dermatol. (2016) 27:361-e91. 10.1111/vde.12358 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

