Phagocytosis: Our Current Understanding of a Universal Biological Process
- PMID: 32582172
- PMCID: PMC7280488
- DOI: 10.3389/fimmu.2020.01066
Phagocytosis: Our Current Understanding of a Universal Biological Process
Abstract
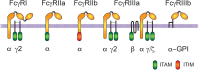
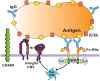
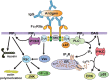
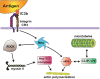
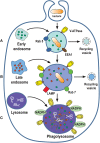
Phagocytosis is a cellular process for ingesting and eliminating particles larger than 0.5 μm in diameter, including microorganisms, foreign substances, and apoptotic cells. Phagocytosis is found in many types of cells and it is, in consequence an essential process for tissue homeostasis. However, only specialized cells termed professional phagocytes accomplish phagocytosis with high efficiency. Macrophages, neutrophils, monocytes, dendritic cells, and osteoclasts are among these dedicated cells. These professional phagocytes express several phagocytic receptors that activate signaling pathways resulting in phagocytosis. The process of phagocytosis involves several phases: i) detection of the particle to be ingested, ii) activation of the internalization process, iii) formation of a specialized vacuole called phagosome, and iv) maturation of the phagosome to transform it into a phagolysosome. In this review, we present a general view of our current understanding on cells, phagocytic receptors and phases involved in phagocytosis.
Keywords: ERK; antibody; complement; immunoglobulin; integrin; neutrophil; phagocytosis.
Copyright © 2020 Uribe-Querol and Rosales.
Figures
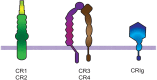
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

