IL-12 Signaling Contributes to the Reprogramming of Neonatal CD8+ T Cells
- PMID: 32582178
- PMCID: PMC7292210
- DOI: 10.3389/fimmu.2020.01089
IL-12 Signaling Contributes to the Reprogramming of Neonatal CD8+ T Cells
Abstract
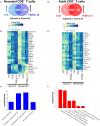
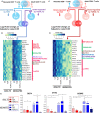
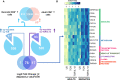
Neonates are highly susceptible to intracellular pathogens, leading to high morbidity and mortality rates. CD8+ T lymphocytes are responsible for the elimination of infected cells. Understanding the response of these cells to normal and high stimulatory conditions is important to propose better treatments and vaccine formulations for neonates. We have previously shown that human neonatal CD8+ T cells overexpress innate inflammatory genes and have a low expression of cytotoxic and cell signaling genes. To investigate the activation potential of these cells, we evaluated the transcriptome of human neonatal and adult naïve CD8+ T cells after TCR/CD28 signals ± IL-12. We found that in neonatal cells, IL-12 signals contribute to the adult-like expression of genes associated with cell-signaling, T-cell cytokines, metabolism, and cell division. Additionally, IL-12 signals contributed to the downregulation of the neutrophil signature transcription factor CEBPE and other immaturity related genes. To validate the transcriptome results, we evaluated the expression of a series of genes by RT-qPCR and the promoter methylation status on independent samples. We found that in agreement with the transcriptome, IL-12 signals contributed to the chromatin closure of neutrophil-like genes and the opening of cytotoxicity genes, suggesting that IL-12 signals contribute to the epigenetic reprogramming of neonatal lymphocytes. Furthermore, high expression of some inflammatory genes was observed in naïve and stimulated neonatal cells, in agreement with the high inflammatory profile of neonates to infections. Altogether our results point to an important contribution of IL-12 signals to the reprogramming of the neonatal CD8+ T cells.
Keywords: CD8+ T cells; IL-12; RNA-sequencing; T cell activation; neonatal T cells; neonatal immunity.
Copyright © 2020 Gutiérrez-Reyna, Cedillo-Baños, Kempis-Calanis, Ramírez-Pliego, Bargier, Puthier, Abad-Flores, Thomas-Chollier, Thieffry, Medina-Rivera, Spicuglia and Santana.
Figures





Similar articles
-
Neonatal CD8+ T-cell differentiation is dependent on interleukin-12.Hum Immunol. 2010 Dec;71(12):1172-9. doi: 10.1016/j.humimm.2010.09.004. Epub 2010 Sep 16. Hum Immunol. 2010. PMID: 20849902
-
CD8+ T Cells from Human Neonates Are Biased toward an Innate Immune Response.Cell Rep. 2016 Nov 15;17(8):2151-2160. doi: 10.1016/j.celrep.2016.10.056. Cell Rep. 2016. PMID: 27851975
-
Maturation of human neonatal CD4+ and CD8+ T lymphocytes into Th1/Th2 effectors.Vaccine. 1998 Aug-Sep;16(14-15):1415-9. doi: 10.1016/s0264-410x(98)00101-7. Vaccine. 1998. PMID: 9711781 Review.
-
CD28, IL-2-independent costimulatory pathways for CD8 T lymphocyte activation.J Immunol. 1999 Aug 1;163(3):1133-42. J Immunol. 1999. PMID: 10415007
-
Cytokine synergy in antigen-independent activation and priming of naive CD8+ T lymphocytes.Crit Rev Immunol. 2009;29(3):219-39. doi: 10.1615/critrevimmunol.v29.i3.30. Crit Rev Immunol. 2009. PMID: 19538136 Review.
Cited by
-
Neonatal Immune Responses to Respiratory Viruses.Front Immunol. 2022 Apr 14;13:863149. doi: 10.3389/fimmu.2022.863149. eCollection 2022. Front Immunol. 2022. PMID: 35493465 Free PMC article. Review.
-
Vaccine-Induced CD8+ T Cell Responses in Children: A Review of Age-Specific Molecular Determinants Contributing to Antigen Cross-Presentation.Front Immunol. 2020 Dec 23;11:607977. doi: 10.3389/fimmu.2020.607977. eCollection 2020. Front Immunol. 2020. PMID: 33424857 Free PMC article. Review.
-
A pilot study of the differentiated landscape of peripheral blood mononuclear cells from children with incomplete versus complete Kawasaki disease.World J Pediatr. 2024 Feb;20(2):189-200. doi: 10.1007/s12519-023-00752-4. Epub 2023 Sep 9. World J Pediatr. 2024. PMID: 37688719 No abstract available.
-
Altered Gut Microbiome and Fecal Immune Phenotype in Early Preterm Infants With Leaky Gut.Front Immunol. 2022 Feb 23;13:815046. doi: 10.3389/fimmu.2022.815046. eCollection 2022. Front Immunol. 2022. PMID: 35280991 Free PMC article.
References
-
- Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, et al. . Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. (1999) 162:3256–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

