Interaction Between Virus-Like Particles (VLPs) and Pattern Recognition Receptors (PRRs) From Dendritic Cells (DCs): Toward Better Engineering of VLPs
- PMID: 32582186
- PMCID: PMC7297083
- DOI: 10.3389/fimmu.2020.01100
Interaction Between Virus-Like Particles (VLPs) and Pattern Recognition Receptors (PRRs) From Dendritic Cells (DCs): Toward Better Engineering of VLPs
Abstract
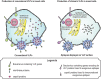
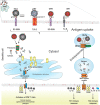
Virus-like particles (VLPs) have been shown to be strong activators of dendritic cells (DCs). DCs are the most potent antigen presenting cells (APCs) and their activation prompts the priming of immunity mediators based on B and T cells. The first step for the activation of DCs is the binding of VLPs to pattern recognition receptors (PRRs) on the surface of DCs, followed by VLP internalization. Like wild-type viruses, VLPs use specific PRRs from the DC; however, these recognition interactions between VLPs and PRRs from DCs have not been thoroughly reviewed. In this review, we focused on the interaction between proteins that form VLPs and PRRs from DCs. Several proteins that form VLP contain glycosylations that allow the direct interaction with PRRs sensing carbohydrates, prompting DC maturation and leading to the development of strong adaptive immune responses. We also discussed how the knowledge of the molecular interaction between VLPs and PRRs from DCs can lead to the smart design of VLPs, whether based on the fusion of foreign epitopes or their chemical conjugation, as well as other modifications that have been shown to induce a stronger adaptive immune response and protection against infectious pathogens of importance in human and veterinary medicine. Finally, we address the use of VLPs as tools against cancer and allergic diseases.
Keywords: allergy; cancer; epitopes; personalized medicine; translational medicine; tropical viral diseases; viruses.
Copyright © 2020 Zepeda-Cervantes, Ramírez-Jarquín and Vaca.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

