Cathelicidins Modulate TLR-Activation and Inflammation
- PMID: 32582207
- PMCID: PMC7296178
- DOI: 10.3389/fimmu.2020.01137
Cathelicidins Modulate TLR-Activation and Inflammation
Abstract
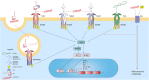
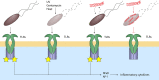
Cathelicidins are short cationic peptides that are part of the innate immune system. At first, these peptides were studied mostly for their direct antimicrobial killing capacity, but nowadays they are more and more appreciated for their immunomodulatory functions. In this review, we will provide a comprehensive overview of the various effects cathelicidins have on the detection of damage- and microbe-associated molecular patterns, with a special focus on their effects on Toll-like receptor (TLR) activation. We review the available literature based on TLR ligand types, which can roughly be divided into lipidic ligands, such as LPS and lipoproteins, and nucleic-acid ligands, such as RNA and DNA. For both ligand types, we describe how direct cathelicidin-ligand interactions influence TLR activation, by for instance altering ligand stability, cellular uptake and receptor interaction. In addition, we will review the more indirect mechanisms by which cathelicidins affect downstream TLR-signaling. To place all this information in a broader context, we discuss how these cathelicidin-mediated effects can have an impact on how the host responds to infectious organisms as well as how these effects play a role in the exacerbation of inflammation in auto-immune diseases. Finally, we discuss how these immunomodulatory activities can be exploited in vaccine development and cancer therapies.
Keywords: DAMPs; LL-37; MAMPs; Toll-like receptors; antimicrobial peptides; cathelicidins; dendritic cells; macrophages.
Copyright © 2020 Scheenstra, van Harten, Veldhuizen, Haagsman and Coorens.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

