Maternal Immune Activation Alters Fetal Brain Development and Enhances Proliferation of Neural Precursor Cells in Rats
- PMID: 32582210
- PMCID: PMC7295982
- DOI: 10.3389/fimmu.2020.01145
Maternal Immune Activation Alters Fetal Brain Development and Enhances Proliferation of Neural Precursor Cells in Rats
Abstract
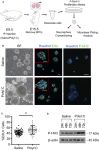
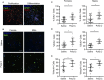
Maternal immune activation (MIA) caused by exposure to pathogens or inflammation during critical periods of neurodevelopment is a major risk factor for behavioral deficits and psychiatric illness in offspring. A spectrum of behavioral abnormalities can be recapitulated in rodents by inducing MIA using the viral mimetic, PolyI:C. Many studies have focused on long-term changes in brain structure and behavioral outcomes in offspring following maternal PolyI:C exposure, but acute changes in prenatal development are not well-characterized. Using RNA-Sequencing, we profiled acute transcriptomic changes in rat conceptuses (decidua along with nascent embryo and placenta) after maternal PolyI:C exposure during early gestation, which enabled us to capture gene expression changes provoked by MIA inclusive to the embryonic milieu. We identified a robust increase in expression of genes related to antiviral inflammation following maternal PolyI:C exposure, and a corresponding decrease in transcripts associated with nervous system development. At mid-gestation, regions of the developing cortex were thicker in fetuses prenatally challenged with PolyI:C, with females displaying a thicker ventricular zone and males a thicker cortical mantle. Along these lines, neural precursor cells (NPCs) isolated from fetal brains prenatally challenged with PolyI:C exhibited a higher rate of self-renewal. Expression of Notch1 and the Notch ligand, delta-like ligand 1, which are both highly implicated in maintenance of NPCs and nervous system development, was increased following PolyI:C exposure. These results suggest that MIA elicits rapid gene expression changes within the conceptus, including repression of neurodevelopmental pathways, resulting in profound alterations in fetal brain development.
Keywords: PolyI:C; fetal brain development; maternal immune activation; neural precursor cells; pregnancy.
Copyright © 2020 Baines, Hillier, Haddad, Rajakumar, Schmid and Renaud.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

