Early Diagnosis and Management of Nitrogen Deficiency in Plants Utilizing Raman Spectroscopy
- PMID: 32582235
- PMCID: PMC7291773
- DOI: 10.3389/fpls.2020.00663
Early Diagnosis and Management of Nitrogen Deficiency in Plants Utilizing Raman Spectroscopy
Abstract
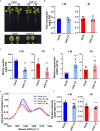
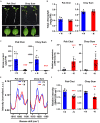
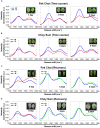
Nutrient deficiency alters growth and development of crop plants and compromises yield. Real-time non-invasive monitoring of the nutritional status of crops would allow timely applications of fertilizers to optimize for growth and yield at different times of the plant's life cycle. Here, we used Raman spectroscopy to characterize Arabidopsis and two varieties of leafy vegetable crops under nitrogen sufficient and deficient conditions. We showed that the 1046 cm-1 Raman peak serves as a specific signature of nitrogen status in planta, which can be used for early diagnosis of nitrogen deficiency in plants before onset of any visible symptoms. Our research can be applied toward crop management for sustainable and precision agriculture.
Keywords: Arabidopsis; Raman spectroscopy; leafy vegetables; nitrate peak; nitrogen deficiency.
Copyright © 2020 Huang, Singh, Park, Chua, Ram and Park.
Figures




References
-
- Blackmer T. M., Schepers J. S., Varvel G. E., Walter-Shea E. A. (1996). Nitrogen deficiency detection using reflected shortwave radiation from irrigated corn canopies. Agron. J. 88 1–5. 10.2134/agronj1996.00021962008800010001x - DOI
-
- Carter G. A. (1994). Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 15 697–703. 10.1080/01431169408954109 - DOI
-
- Cataldo D. A., Maroon M., Schrader L. E., Youngs V. L. (1975). Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 6 71–80. 10.1080/00103627509366547 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

