Enzymatic Functions for Toll/Interleukin-1 Receptor Domain Proteins in the Plant Immune System
- PMID: 32582284
- PMCID: PMC7282519
- DOI: 10.3389/fgene.2020.00539
Enzymatic Functions for Toll/Interleukin-1 Receptor Domain Proteins in the Plant Immune System
Abstract
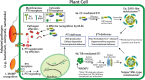
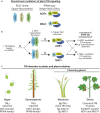
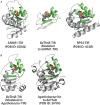
Rationally engineered improvements to crop plants will be needed to keep pace with increasing demands placed on agricultural systems by population growth and climate change. Engineering of plant immune systems provides an opportunity to increase yields by limiting losses to pathogens. Intracellular immune receptors are commonly used as agricultural disease resistance traits. Despite their importance, how intracellular immune receptors confer disease resistance is still unknown. One major class of immune receptors in dicots contains a Toll/Interleukin-1 Receptor (TIR) domain. The mechanisms of TIR-containing proteins during plant immunity have remained elusive. The TIR domain is an ancient module found in archaeal, bacterial and eukaryotic proteins. In animals, TIR domains serve a structural role by generating innate immune signaling complexes. The unusual animal TIR-protein, SARM1, was recently discovered to function instead as an enzyme that depletes cellular NAD+ (nicotinamide adenine dinucleotide) to trigger axonal cell death. Two recent reports have found that plant TIR proteins also have the ability to cleave NAD+. This presents a new paradigm from which to consider how plant TIR immune receptors function. Here, we will review recent reports of the structure and function of TIR-domain containing proteins. Intriguingly, it appears that TIR proteins in all kingdoms may use similar enzymatic mechanisms in a variety of cell death and immune pathways. We will also discuss TIR structure-function hypotheses in light of the recent publication of the ZAR1 resistosome structure. Finally, we will explore the evolutionary context of plant TIR-containing proteins and their downstream signaling components across phylogenies and the functional implications of these findings.
Keywords: NADase; NLR; TIR; Toll/interleukin-1 receptor; innate immunity.
Copyright © 2020 Bayless and Nishimura.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

