HMTase Inhibitors as a Potential Epigenetic-Based Therapeutic Approach for Friedreich's Ataxia
- PMID: 32582297
- PMCID: PMC7291394
- DOI: 10.3389/fgene.2020.00584
HMTase Inhibitors as a Potential Epigenetic-Based Therapeutic Approach for Friedreich's Ataxia
Abstract
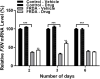
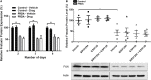
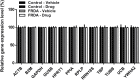
Friedreich's ataxia (FRDA) is a progressive neurodegenerative disorder caused by a homozygous GAA repeat expansion mutation in intron 1 of the frataxin gene (FXN), which instigates reduced transcription. As a consequence, reduced levels of frataxin protein lead to mitochondrial iron accumulation, oxidative stress, and ultimately cell death; particularly in dorsal root ganglia (DRG) sensory neurons and the dentate nucleus of the cerebellum. In addition to neurological disability, FRDA is associated with cardiomyopathy, diabetes mellitus, and skeletal deformities. Currently there is no effective treatment for FRDA and patients die prematurely. Recent findings suggest that abnormal GAA expansion plays a role in histone modification, subjecting the FXN gene to heterochromatin silencing. Therefore, as an epigenetic-based therapy, we investigated the efficacy and tolerability of two histone methyltransferase (HMTase) inhibitor compounds, BIX0194 (G9a-inhibitor) and GSK126 (EZH2-inhibitor), to specifically target and reduce H3K9me2/3 and H3K27me3 levels, respectively, in FRDA fibroblasts. We show that a combination treatment of BIX0194 and GSK126, significantly increased FXN gene expression levels and reduced the repressive histone marks. However, no increase in frataxin protein levels was observed. Nevertheless, our results are still promising and may encourage to investigate HMTase inhibitors with other synergistic epigenetic-based therapies for further preliminary studies.
Keywords: FRDA; FXN; Friedreich ataxia; GAA repeat; HMTase inhibitor; frataxin.
Copyright © 2020 Sherzai, Valle, Perry, Kalef-Ezra, Al-Mahdawi, Pook and Anjomani Virmouni.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

