In silico design of two novel fusion proteins, p28-IL-24 and p28-M4, targeted to breast cancer cells
- PMID: 32582360
- PMCID: PMC7306244
- DOI: 10.4103/1735-5362.283820
In silico design of two novel fusion proteins, p28-IL-24 and p28-M4, targeted to breast cancer cells
Abstract
Background and purpose: An anticancer peptide P28, has shown to be cytolethal on various cancer cells including breast cancer. Moreover, p28 can be also used as a targeting moiety in the structure of fusion proteins. IL-24 (or its truncated form, M4) is a cytokine with anticancer activity against a wide range of tumor cells. We aimed at production of a fusion protein consisted of p28 and either IL-24 or M4 to target breast cancer. However, selection of a proper linker to join the two moieties without intervening each other's function is a key factor in the construction of fusion proteins. In the present study, the impact of different linkers on construction of the two chimeric proteins (p28-IL-24 and p28-M4) was assessed in silico.
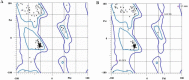
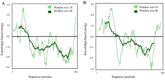
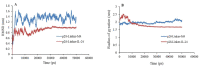
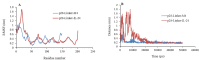
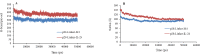
Experimental approach: After selection of some linkers with different lengths and characteristics, a small library of the chimeric proteins was created and assessed. Furthermore, following selection of the most suitable linker, the three-dimensional structures and dynamic behavior of both fusion proteins were evaluated by homology modeling and molecular dynamic simulation, respectively.
Findings / results: Based on the results, a rigid linker having the peptide sequences of AEAAAKEAAAKA showed highest freedom of action for both moieties.
Conclusion and implications: Between the p28-IL-24 and p28-M4 fusion proteins, the former showed better stability as well as solubility and might show stronger anticancer effects in vitro and in vivo, because its peptide moieties showed to exert their activities freely.
Keywords: Breast cancer; Homology modeling; IL-24; Molecular Dynamic Simulation; fusion protein; p28.
Copyright: © 2020 Research in Pharmaceutical Sciences.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
-
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends-an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. DOI: 10.1158/1055-9965.EPI-15-0578. - PubMed
-
- Saini RK, Chouhan R, Bagri LP, Bajpai AK. Strategies of targeting tumors and cancers. J Can Res Updates. 2012;1(1):129–152. DOI: 10.6000/1929-2279.2012.01.01.19.
LinkOut - more resources
Full Text Sources
