RENATA study-Latin American prospective experience: clinical outcome of patients treated with palbociclib in hormone receptor-positive metastatic breast cancer-real-world use
- PMID: 32582373
- PMCID: PMC7302883
- DOI: 10.3332/ecancer.2020.1058
RENATA study-Latin American prospective experience: clinical outcome of patients treated with palbociclib in hormone receptor-positive metastatic breast cancer-real-world use
Abstract
Background: In hormone receptor-positive, HER-2 negative (HR+/HER2-) advanced breast cancer (ABC) endocrine therapy (ET) plus cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) in first and second line improved progression-free survival (PFS), overall response rate (ORR) and clinical benefit rate (CB) without deterioration in quality of life compared with ET alone. In addition, recent data showed improvement in overall survival (OS) for premenopausal women in first line setting and for different subgroups of patients in second line. Since 2015, in Argentina, the combination of ET with CDK4/6i is a standard of care in HR+/HER2- ABC.
Methods: We carried out a prospective analysis of real-world use of palbociclib with ET in HR+/HER2- ABC patients who received treatment between October 2015 and August 2019 in two private institutes from Buenos Aires, Argentina. The aims of the study were to determine efficacy and safety of patients treated with ET and palbociclib, describe patient profile and treatment strategy beyond progression.
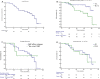
Results: One-hundred and twenty-eight patients were included in the final analysis. Main baseline characteristics include, median age 57 years, 20% were premenopausal women, 44% had visceral metastasis and 26% bone only disease. More than half of patients had two or more metastatic sites, 44.4% had performance status 1, and most of them (59.4%) were treated with palbociclib in first-line setting. Palbociclib was preferentially associated with aromatase inhibitors in 63.9% of patients, and with fulvestrant in the remaining. All premenopausal women received ovarian suppression or ovarian ablation (OS/OA). The median PFS was 36.7 months in first line and 24.2 months in second line. The ORR was 45.3% and 25.0% in first and second line, respectively. The median OS in the entire population was not reached. Half of patients did not require dose interruption and/or delay, dose reduction was required in 15% of patients and almost no patients required drug discontinuation (2.0%). With regard to safety, 55% of patients developed grade 3-4 adverse events, 20% neutropenia grade 3-4, and 7% febrile neutropenia. Infections were presented in one out of three patients, mostly uncomplicated.
Conclusions: This is the first prospective evidence of real-world use of palbociclib in a Latin American population. We found similar outcomes to the PALOMA-2 and PALOMA-3 randomised trials and Real-World Data already published, with lower incidence of side effects and treatment discontinuation, but with higher incidence of febrile neutropenia.
Keywords: Palbociclib; advanced breast cancer; hormone receptor-positive; overall survival; progression-free survival; real-world.
© the authors; licensee ecancermedicalscience.
Conflict of interest statement
There are no conflicts of interest.
Figures

References
-
- Observatorio Global de Cáncer de la OMS [ https://www.argentina.gob.ar/salud/instituto-nacional-del-cancer/estadis...]
-
- Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptorepositive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. - DOI - PubMed
-
- Blum J, Tripathy D, Bardia A, et al. POLARIS: palbociclib in hormone receptor-positive advanced breast cancer: a prospective multicenter noninterventional study. ESMO Congress 2018 Poster 344P.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
