The PANoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis)
- PMID: 32582562
- PMCID: PMC7283380
- DOI: 10.3389/fcimb.2020.00238
The PANoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis)
Abstract
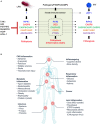
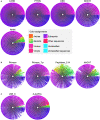
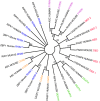
Programmed cell death is regulated by evolutionarily conserved pathways that play critical roles in development and the immune response. A newly recognized pathway for proinflammatory programmed cell death called PANoptosis is controlled by a recently identified cytoplasmic multimeric protein complex named the PANoptosome. The PANoptosome can engage, in parallel, three key modes of programmed cell death-pyroptosis, apoptosis, and necroptosis. The PANoptosome components have been implicated in a wide array of human diseases including autoinflammatory diseases, neurodegenerative diseases, cancer, microbial infections, and metabolic diseases. Here, we review putative components of the PANoptosome and present a phylogenetic analysis of their molecular domains and interaction motifs that support complex assembly. We also discuss genetic data that suggest PANoptosis is coordinated by scaffolding and catalytic functions of the complex components and propose mechanistic models for PANoptosome assembly. Overall, this review presents potential mechanisms governing PANoptosis based on evolutionary analysis of the PANoptosome components.
Keywords: ASC; PANoptosis; PANoptosome; RIPK1; RIPK3; ZBP1; caspase-1; caspase-8.
Copyright © 2020 Samir, Malireddi and Kanneganti.
Figures
References
-
- Aganna E., Martinon F., Hawkins P. N., Ross J. B., Swan D. C., Booth D. R., et al. (2002). Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 46, 2445–2452. 10.1002/art.10509 - DOI - PubMed
-
- Aksentijevich I., Nowak M., Mallah M., Chae J. J., Watford W. T., Hofmann S. R., et al. (2002). De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 46, 3340–3348. 10.1002/art.10688 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

