Minor Histocompatibility Antigen-Specific T Cells
- PMID: 32582592
- PMCID: PMC7283489
- DOI: 10.3389/fped.2020.00284
Minor Histocompatibility Antigen-Specific T Cells
Abstract
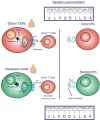
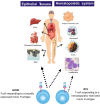
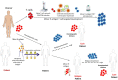
Minor Histocompatibility (H) antigens are major histocompatibility complex (MHC)/Human Leukocyte Antigen (HLA)-bound peptides that differ between allogeneic hematopoietic stem cell transplantation (HCT) recipients and their donors as a result of genetic polymorphisms. Some minor H antigens can be used as therapeutic T cell targets to augment the graft-vs.-leukemia (GVL) effect in order to prevent or manage leukemia relapse after HCT. Graft engineering and post-HCT immunotherapies are being developed to optimize delivery of T cells specific for selected minor H antigens. These strategies have the potential to reduce relapse risk and thereby permit implementation of HCT approaches that are associated with less toxicity and fewer late effects, which is particularly important in the growing and developing pediatric patient. Most minor H antigens are expressed ubiquitously, including on epithelial tissues, and can be recognized by donor T cells following HCT, leading to graft-vs.-host disease (GVHD) as well as GVL. However, those minor H antigens that are expressed predominantly on hematopoietic cells can be targeted for selective GVL. Once full donor hematopoietic chimerism is achieved after HCT, hematopoietic-restricted minor H antigens are present only on residual recipient malignant hematopoietic cells, and these minor H antigens serve as tumor-specific antigens for donor T cells. Minor H antigen-specific T cells that are delivered as part of the donor hematopoietic stem cell graft at the time of HCT contribute to relapse prevention. However, in some cases the minor H antigen-specific T cells delivered with the graft may be quantitatively insufficient or become functionally impaired over time, leading to leukemia relapse. Following HCT, adoptive T cell immunotherapy can be used to treat or prevent relapse by delivering large numbers of donor T cells targeting hematopoietic-restricted minor H antigens. In this review, we discuss minor H antigens as T cell targets for augmenting the GVL effect in engineered HCT grafts and for post-HCT immunotherapy. We will highlight the importance of these developments for pediatric HCT.
Keywords: T cell immunotherapy; graft engineering; graft-vs.-leukemia; hematopoietic stem cell transplantation; leukemia; minor histocompatibility antigen; pediatric; polymorphism.
Copyright © 2020 Summers, Sheth and Bleakley.
Figures

References
-
- Oliansky DM, Camitta B, Gaynon P, Nieder ML, Parsons SK, Pulsipher MA, et al. Role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: update of the 2005 evidence-based review. Biol Blood Marrow Transplant. (2012) 18:505–22. 10.1016/j.bbmt.2011.12.585 - DOI - PubMed
-
- Cornelissen JJ, van Putten WL, Verdonck LF, Theobald M, Jacky E, Daenen SM, et al. . Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. (2007) 109:3658–66. 10.1182/blood-2006-06-025627 - DOI - PubMed
-
- Cornelissen JJ, van der Holt B, Verhoef GE, van't Veer MB, van Oers MH, Schouten HC, et al. . Myeloablative allogeneic versus autologous stem cell transplantation in adult patients with acute lymphoblastic leukemia in first remission: a prospective sibling donor versus no-donor comparison. Blood. (2009) 113:1375–82. 10.1182/blood-2008-07-168625 - DOI - PubMed
-
- Shouval R, Fein JA, Labopin M, Kroger N, Duarte RF, Bader P, et al. . Outcomes of allogeneic haematopoietic stem cell transplantation from HLA-matched and alternative donors: a European society for blood and marrow transplantation registry retrospective analysis. Lancet Haematol. (2019) 6:e573–e584. 10.1016/S2352-3026(19)30158-9 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

