Kidney Ischemia-Reperfusion Elicits Acute Liver Injury and Inflammatory Response
- PMID: 32582723
- PMCID: PMC7280447
- DOI: 10.3389/fmed.2020.00201
Kidney Ischemia-Reperfusion Elicits Acute Liver Injury and Inflammatory Response
Abstract
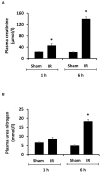
Ischemia-reperfusion (IR) is a common risk factor that causes acute kidney injury (AKI). AKI is associated with dysfunction of other organs also known as distant organ injury. The liver function is often compromised in patients with AKI and in animal models. However, the underlying mechanisms are not fully understood. Inflammatory response plays an important role in IR-induced tissue injury. Although increased proinflammatory cytokines have been detected in the kidney and the distant organs after renal IR, their original sources remain uncertain. In the present study, we investigated the acute effect of renal IR on hepatic inflammatory cytokine expression and the mechanism involved. Sprague-Dawley rats that were subjected to renal IR (ischemia for 45 min followed by reperfusion for 1 h or 6 h) had increased plasma levels of creatinine, urea, and transaminases, indicating kidney and liver injuries. There was a significant increase in the expression of proinflammatory cytokine mRNA (MCP-1, TNF-α, IL-6) in the kidney and liver in rats with renal IR. This was accompanied by a significant increase in proinflammatory cytokine protein levels in the plasma, kidney, and liver. Activation of a nuclear transcription factor kappa B (NF-κB) was detected in the liver after renal IR. The inflammatory foci and an increased myeloperoxidase (MPO) activity were detected in the liver after renal IR, indicating hepatic inflammatory response and leukocyte infiltration. These results suggest that renal IR can directly activate NF-κB and induce acute production of proinflammatory cytokines in the liver. Renal IR-induced hepatic inflammatory response may contribute to impaired liver function and systemic inflammation.
Keywords: acute kidney injury; inflammation; ischemia-reperfusion; kidney; liver.
Copyright © 2020 Shang, Madduma Hewage, Wijerathne, Siow, Isaak and O.
Figures








References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

