Hsp60 Post-translational Modifications: Functional and Pathological Consequences
- PMID: 32582761
- PMCID: PMC7289027
- DOI: 10.3389/fmolb.2020.00095
Hsp60 Post-translational Modifications: Functional and Pathological Consequences
Abstract
Hsp60 is a chaperone belonging to the Chaperonins of Group I and typically functions inside mitochondria in which, together with the co-chaperonin Hsp10, maintains protein homeostasis. In addition to this canonical role, Hsp60 plays many others beyond the mitochondria, for instance in the cytosol, plasma-cell membrane, extracellular space, and body fluids. These non-canonical functions include participation in inflammation, autoimmunity, carcinogenesis, cell replication, and other cellular events in health and disease. Thus, Hsp60 is a multifaceted molecule with a wide range of cellular and tissue locations and functions, which is noteworthy because there is only one hsp60 gene. The question is by what mechanism this protein can become multifaceted. Likely, one factor contributing to this diversity is post-translational modification (PTM). The amino acid sequence of Hsp60 contains many potential phosphorylation sites, and other PTMs are possible such as O-GlcNAcylation, nitration, acetylation, S-nitrosylation, citrullination, oxidation, and ubiquitination. The effect of some of these PTMs on Hsp60 functions have been examined, for instance phosphorylation has been implicated in sperm capacitation, docking of H2B and microtubule-associated proteins, mitochondrial dysfunction, tumor invasiveness, and delay or facilitation of apoptosis. Nitration was found to affect the stability of the mitochondrial permeability transition pore, to inhibit folding ability, and to perturb insulin secretion. Hyperacetylation was associated with mitochondrial failure; S-nitrosylation has an impact on mitochondrial stability and endothelial integrity; citrullination can be pro-apoptotic; oxidation has a role in the response to cellular injury and in cell migration; and ubiquitination regulates interaction with the ubiquitin-proteasome system. Future research ought to determine which PTM causes which variations in the Hsp60 molecular properties and functions, and which of them are pathogenic, causing chaperonopathies. This is an important topic considering the number of acquired Hsp60 chaperonopathies already cataloged, many of which are serious diseases without efficacious treatment.
Keywords: Hsp60; canonical functions; chaperonin; chaperonopathies; non-canonical functions; post-translation modification.
Copyright © 2020 Caruso Bavisotto, Alberti, Vitale, Paladino, Campanella, Rappa, Gorska, Conway de Macario, Cappello, Macario and Marino Gammazza.
Figures
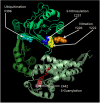
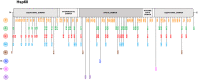
Similar articles
-
Molecular mechanisms in chaperonopathies: clues to understanding the histopathological abnormalities and developing novel therapies.J Pathol. 2020 Jan;250(1):9-18. doi: 10.1002/path.5349. Epub 2019 Nov 22. J Pathol. 2020. PMID: 31579936 Review.
-
Curcumin Affects HSP60 Folding Activity and Levels in Neuroblastoma Cells.Int J Mol Sci. 2020 Jan 19;21(2):661. doi: 10.3390/ijms21020661. Int J Mol Sci. 2020. PMID: 31963896 Free PMC article.
-
A Cell Model for HSP60 Deficiencies: Modeling Different Levels of Chaperonopathies Leading to Oxidative Stress and Mitochondrial Dysfunction.Methods Mol Biol. 2019;1873:225-239. doi: 10.1007/978-1-4939-8820-4_14. Methods Mol Biol. 2019. PMID: 30341613
-
Doxorubicin anti-tumor mechanisms include Hsp60 post-translational modifications leading to the Hsp60/p53 complex dissociation and instauration of replicative senescence.Cancer Lett. 2017 Jan 28;385:75-86. doi: 10.1016/j.canlet.2016.10.045. Epub 2016 Nov 9. Cancer Lett. 2017. PMID: 27836734
-
Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy.Cancer Biol Ther. 2008 Jun;7(6):801-9. doi: 10.4161/cbt.7.6.6281. Epub 2008 May 13. Cancer Biol Ther. 2008. PMID: 18497565 Review.
Cited by
-
Arbovirus-vector protein interactomics identifies Loquacious as a co-factor for dengue virus replication in Aedes mosquitoes.PLoS Pathog. 2022 Sep 8;18(9):e1010329. doi: 10.1371/journal.ppat.1010329. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36074777 Free PMC article.
-
Lung Single-Cell Transcriptomics Offers Insights into the Pulmonary Interstitial Toxicity Caused by Silica Nanoparticles.Environ Health (Wash). 2024 Jul 1;2(11):786-801. doi: 10.1021/envhealth.4c00052. eCollection 2024 Nov 15. Environ Health (Wash). 2024. PMID: 39568699 Free PMC article.
-
Heat shock proteins as hallmarks of cancer: insights from molecular mechanisms to therapeutic strategies.J Hematol Oncol. 2024 Sep 4;17(1):81. doi: 10.1186/s13045-024-01601-1. J Hematol Oncol. 2024. PMID: 39232809 Free PMC article. Review.
-
Targeting PRMT3 impairs methylation and oligomerization of HSP60 to boost anti-tumor immunity by activating cGAS/STING signaling.Nat Commun. 2024 Sep 10;15(1):7930. doi: 10.1038/s41467-024-52170-3. Nat Commun. 2024. PMID: 39256398 Free PMC article.
-
KBTBD11, encoding a novel PPARγ target gene, is involved in NFATc1 proteolysis by interacting with HSC70 and HSP60.Sci Rep. 2022 Nov 24;12(1):20273. doi: 10.1038/s41598-022-24929-5. Sci Rep. 2022. PMID: 36434268 Free PMC article.
References
-
- Barazi H. O., Zhou L., Templeton N. S., Krutzsch H. C., Roberts D. D. (2002). Identification of heat shock protein 60 as a molecular mediator of alpha 3 beta 1 integrin activation. Cancer Res. 62 1541–1548. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

