Oleic and Linoleic Acids Induce the Release of Neutrophil Extracellular Traps via Pannexin 1-Dependent ATP Release and P2X1 Receptor Activation
- PMID: 32582772
- PMCID: PMC7291836
- DOI: 10.3389/fvets.2020.00260
Oleic and Linoleic Acids Induce the Release of Neutrophil Extracellular Traps via Pannexin 1-Dependent ATP Release and P2X1 Receptor Activation
Abstract
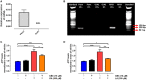
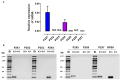
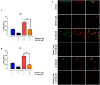
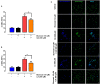
Non-esterified fatty acids (NEFAs) such as oleic acid (OA) and linoleic acid (LA) are associated with a higher incidence of infectious diseases such as metritis and mastitis during the bovine peripartum. Fatty acids can induce an increase in the release of ATP, and changes in the expression levels of purinergic receptors in bovine polymorphonuclears (PMN) during peripartum have also been reported. PMN respond to inflammatory processes with production of ROS, release of proteolytic and bactericidal proteins, and formation of neutrophil extracellular traps (NETs). NETs formation is known to require ATP production through glycolysis. Studies have shown that the above-mentioned metabolic changes alter innate immune responses, particularly in PMN. We hypothesized that NEFAs induce the formation of NETs through ATP release by Pannexin 1 and activation of purinergic receptors. In this study, we found that OA and LA induce NET formation and extracellular ATP release. Carbenoxolone, a pannexin-1 (PANX1) inhibitor, reduced OA- and LA-induced ATP release. We also found that P2X1, P2X4, P2X5, P2X7, and PANX1 were expressed at the mRNA level in bovine PMN. Additionally, NEFA-induced NET formation was completely abolished with exposure to NF449, a P2X1 antagonist, and partially inhibited by treatment with etomoxir, an inhibitor of fatty acid oxidation (FAO). Our results suggest that OA and LA induce NET formation and ATP release via PANX1 and activation of P2X1. These new data contribute to explaining the effects of NEFA high concentrations during the transition period of dairy cattle and further understanding of pro-inflammatory effects and outcome of postpartum diseases.
Keywords: ATP; PMN; neutrophil extracellular trap; non-esterified fatty acids; purinergic receptor.
Copyright © 2020 Alarcón, Manosalva, Quiroga, Belmar, Álvarez, Díaz, Taubert, Hermosilla, Carretta, Burgos and Hidalgo.
Figures



Similar articles
-
Besnoitia besnoiti-induced neutrophil clustering and neutrophil extracellular trap formation depend on P2X1 purinergic receptor signaling.Front Immunol. 2023 Oct 3;14:1244068. doi: 10.3389/fimmu.2023.1244068. eCollection 2023. Front Immunol. 2023. PMID: 37854595 Free PMC article.
-
Mitochondria-derived ATP participates in the formation of neutrophil extracellular traps induced by platelet-activating factor through purinergic signaling in cows.Dev Comp Immunol. 2020 Dec;113:103768. doi: 10.1016/j.dci.2020.103768. Epub 2020 Jul 18. Dev Comp Immunol. 2020. PMID: 32692996
-
ATP Purinergic Receptor P2X1-Dependent Suicidal NETosis Induced by Cryptosporidium parvum under Physioxia Conditions.Biology (Basel). 2022 Mar 14;11(3):442. doi: 10.3390/biology11030442. Biology (Basel). 2022. PMID: 35336816 Free PMC article.
-
The P2X7 Receptor in Inflammatory Diseases: Angel or Demon?Front Pharmacol. 2018 Feb 6;9:52. doi: 10.3389/fphar.2018.00052. eCollection 2018. Front Pharmacol. 2018. PMID: 29467654 Free PMC article. Review.
-
The two faces of pannexins: new roles in inflammation and repair.J Inflamm Res. 2018 Jun 21;11:273-288. doi: 10.2147/JIR.S128401. eCollection 2018. J Inflamm Res. 2018. PMID: 29950881 Free PMC article. Review.
Cited by
-
Effects of Hormone, NEFA and SCFA on the Migration of Neutrophils and the Formation of Neutrophil Extracellular Traps in Dairy Cows.Animals (Basel). 2022 May 6;12(9):1190. doi: 10.3390/ani12091190. Animals (Basel). 2022. PMID: 35565616 Free PMC article.
-
Glycolysis, monocarboxylate transport, and purinergic signaling are key events in Eimeria bovis-induced NETosis.Front Immunol. 2022 Aug 11;13:842482. doi: 10.3389/fimmu.2022.842482. eCollection 2022. Front Immunol. 2022. PMID: 36032127 Free PMC article.
-
A novel definition and treatment of hyperinflammation in COVID-19 based on purinergic signalling.Purinergic Signal. 2022 Mar;18(1):13-59. doi: 10.1007/s11302-021-09814-6. Epub 2021 Nov 10. Purinergic Signal. 2022. PMID: 34757513 Free PMC article. Review.
-
Neutrophil swarming: Is a good offense the best defense?iScience. 2023 Aug 17;26(9):107655. doi: 10.1016/j.isci.2023.107655. eCollection 2023 Sep 15. iScience. 2023. PMID: 37670784 Free PMC article. Review.
-
Pancreatic melatonin enhances anti-tumor immunity in pancreatic adenocarcinoma through regulating tumor-associated neutrophils infiltration and NETosis.Acta Pharm Sin B. 2023 Apr;13(4):1554-1567. doi: 10.1016/j.apsb.2023.01.020. Epub 2023 Feb 2. Acta Pharm Sin B. 2023. PMID: 37139434 Free PMC article.
References
LinkOut - more resources
Full Text Sources

