Histological, Immunological, and Genetic Analysis of Feline Chronic Gingivostomatitis
- PMID: 32582783
- PMCID: PMC7283503
- DOI: 10.3389/fvets.2020.00310
Histological, Immunological, and Genetic Analysis of Feline Chronic Gingivostomatitis
Abstract
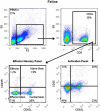
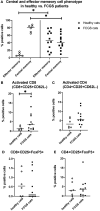
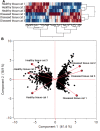
Feline chronic gingivostomatitis (FCGS) is an immune-mediated inflammatory condition affecting the oral mucosa that results in substantial pain and suffering. The goal of this study was to complete an in-depth immunohistochemistry analysis of affected FCGS mucosa, to perform and compare immune cell phenotypes in the blood of FCGS and healthy controls cats, and to determine a transcriptomic profile of the affected and normal oral mucosa of FCGS cats. We hypothesized that cats with FCGS would have circulating activated CD8+ T cells and that tissues would be infiltrated with activated B and T cells with a highly proinflammatory transcriptome. We found that oral mucosal tissues from cats with FCGS have high tissue infiltration of B cells and that T cells include both CD4+ and CD8+ lymphocytes. Cells positive for CD25 (IL2 receptor, indicative of lymphocyte activation) and FOXP3 (indicative of regulatory T cells) were scattered throughout the mucosa. Compared to healthy individuals, cats with FCGS had high circulating CD8+ effector memory cells with a concurrent decrease in central memory cells and evidence of circulating activated CD8+ T cells (CD25+, CD62L-). Gene expression in the affected tissues was enriched for genes associated with T-cell signaling, cell adhesion molecules, leukocyte migration, inflammatory signaling pathways, extracellular matrix-receptor interactions, cytokine-cytokine receptor interactions, and natural killer cell-mediated cytotoxicity, among others. These data are essential to understand disease pathogenesis, to inform mechanism of action studies for future and current therapies, and to help select prognostic biomarkers and potency assays for stem cell treatment of FCGS.
Keywords: chronic feline stomatitis; feline oral mucosal disease; immune-mediated oral mucosal inflammation; immunohistochemistry of feline stomatitis; immunophenotyping of FCGS; transcriptome of chronic gingivostomatitis.
Copyright © 2020 Vapniarsky, Simpson, Arzi, Taechangam, Walker, Garrity, Bulkeley and Borjesson.
Figures

References
-
- Quimby JM, Elston T, Hawley J, Brewer M, Miller A, Lappin MR. Evaluation of the association of Bartonella species, feline herpesvirus 1, feline calicivirus, feline leukemia virus and feline immunodeficiency virus with chronic feline gingivostomatitis. J Feline Med Surg. (2008) 10:66–72. 10.1016/j.jfms.2007.05.007 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

