How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies
- PMID: 32582924
- PMCID: PMC7441168
- DOI: 10.1182/blood.2019004000
How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies
Abstract
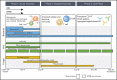
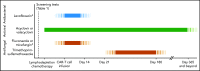
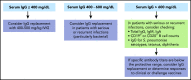
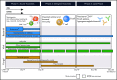
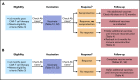
Adoptive immunotherapy using B-cell-targeted chimeric antigen receptor (CAR)-modified T cells to treat hematologic malignancies is transforming cancer care for patients with refractory or relapsed diseases. Recent and anticipated regulatory approval for products targeting acute lymphoblastic leukemia, lymphomas, and multiple myeloma have led to global implementation of these novel treatments. The rapidity of commercial utilization of CAR-T-cell therapy has created a largely unexplored gap in patient supportive-care approaches. Such approaches are critical in these complex patients given their high net state of immunosuppression prior to CAR-T-cell infusion coupled with unique acute and persistent insults to their immune function after CAR-T-cell infusion. In this "How I Treat" article, we focus on key questions that arise during 3 phases of management for patients receiving CD19-targeted CAR-T cells: pre CAR-T-cell infusion, immediate post CAR-T-cell infusion, and long-term follow-up. A longitudinal patient case is presented for each phase to highlight fundamental issues including infectious diseases screening, antimicrobial prophylaxis, immunoglobulin supplementation, risk factors for infection, and vaccination. We hope this discussion will provide a framework for institutions and health care providers to formulate their own approach to preventing infections in light of the paucity of data specific to this treatment modality.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: J.A.H. has served as a consultant for Nohla Therapeutics, Inc, Amplyx, and Gilead Sciences, and has received research support from Nohla Therapeutics, Karius, and Takeda (formerly Shire). S.K.S. declares no competing financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

