Lapatinib‑induced inhibition of ovarian function is counteracted by the STAT3 pathway both in vivo and in vitro
- PMID: 32582968
- PMCID: PMC7388577
- DOI: 10.3892/or.2020.7660
Lapatinib‑induced inhibition of ovarian function is counteracted by the STAT3 pathway both in vivo and in vitro
Erratum in
-
[Corrigendum] Lapatinib‑induced inhibition of ovarian function is counteracted by the STAT3 pathway both in vivo and in vitro.Oncol Rep. 2021 Jul;46(1):123. doi: 10.3892/or.2021.8074. Epub 2021 May 13. Oncol Rep. 2021. PMID: 33982768 Free PMC article.
Abstract
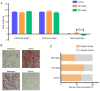
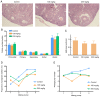
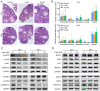
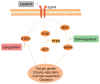
The present study was designed to ascertain whether lapatinib, a tyrosine kinase inhibitor of epidermal growth factor receptor (EGFR), affects ovarian reserve and fertility potential in a mouse model. Female C57BL/6 mice were treated with either vehicle or lapatinib (100 or 200 mg/kg/day orally) for 4 weeks, after which body weight, vaginal smears, follicle numbers, serum anti‑Müllerian hormone (AMH) levels and mating outcomes were analyzed to assess the ovarian reserve and reproductive function. Slices from the ovaries of 4‑week‑old mice were cultured with lapatinib (0, 5 or 10 µM) for 24 and 48 h, and protein expression levels were assessed to validate the changes in signaling pathways. The results indicated that mice treated with 200 mg/kg lapatinib showed a slight decrease in body weight compared to those treated with vehicle or 100 mg/kg lapatinib. There was no statistical difference in estrous cyclicity among the three groups. No significant difference was observed in follicle numbers, AMH levels, histological morphologies of the ovaries or mating outcomes in the three groups of mice. Western blotting and immunohistochemical staining of the EGF receptor and its main downstream signaling pathways showed decreased phosphorylation of EGFR and mitogen‑activated protein kinase (MAPK)3/1 and increased phosphorylation of signal transducers and activators of transcription (STAT)3 in the lapatinib‑treated groups compared to the control group. Our study suggests that lapatinib has little effect on ovarian reserve and reproductive function in a mouse model. This lack of effect of lapatinib on ovarian function may be due to the activation of the STAT3 signaling pathway that counteracts the inhibitory effects of lapatinib on EGF receptors.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

