E2F7, regulated by miR‑30c, inhibits apoptosis and promotes cell cycle of prostate cancer cells
- PMID: 32582990
- PMCID: PMC7388350
- DOI: 10.3892/or.2020.7659
E2F7, regulated by miR‑30c, inhibits apoptosis and promotes cell cycle of prostate cancer cells
Abstract
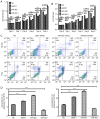
Prostate cancer (PCa) remains a leading cause of mortality among men in the United States and Western Europe. The molecular mechanism of PCa pathogenesis has not been fully elucidated. In the present study, the expression profile of E2F transcription factor 7 (E2F7) in PCa was examined using immunohistochemistry and reverse transcription‑quantitative PCR, whilst cell cycle progression and apoptosis were determined using fluorescent cell activated sorting techniques. Cell viability was measured using Cell Counting Kit‑8 in loss‑ and gain‑of‑function studies. Dual‑luciferase reporter assay was used to verify if E2F7 was one of the potential targets of miR‑30c. The staining score of E2F7 of PCa tissues was found to be notably higher compared with that of adjacent normal tissues. Suppression of E2F7 expression in PCa cell lines led to significantly reduced proliferation rates, increased proportion of cells in the G1 phase of the cell cycle and higher apoptotic rates compared with those in negative control groups. Dual‑luciferase reporter assay revealed E2F7 to be one of the binding targets of microRNA (miR)‑30c. In addition, transfection of miR‑30c mimics into PCa cells resulted in reduced cell viability, increased proportion of cells in the G1 phase and higher apoptotic rates. By contrast, transfection with the miR‑30c inhibitor led to lower apoptosis rates of PCa cells compared with negative control groups, whilst E2F7 siRNA co‑transfection reversed stimulatory effects of miR‑30c inhibitors on cell viability. In addition, the expression of cyclin‑dependent kinase inhibitor p21 were found to be upregulated by transfection with either E2F7 siRNA or miR‑30c mimics into PCa cells. In conclusion, the present study suggested that E2F7 may be positively associated with PCa cell proliferation by inhibiting p21, whereas E2F7 is in turn under regulation by miR‑30c. These observations suggest the miR‑30c/E2F7/p21 axis to be a viable therapeutic target for PCa.
Keywords: prostate cancer; microRNA‑30c; E2F transcription factor 7; cell cycle; apoptosis; proliferation.
Figures







References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;60:277–300.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

