Comparison of the therapeutic effects of lobaplatin and carboplatin on retinoblastoma in vitro and in vivo
- PMID: 32582992
- PMCID: PMC7384850
- DOI: 10.3892/ijo.2020.5085
Comparison of the therapeutic effects of lobaplatin and carboplatin on retinoblastoma in vitro and in vivo
Abstract
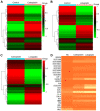
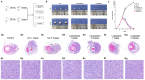
Retinoblastoma (RB) is one of the most aggressive malignancies affecting infants and children. Platinum drugs are commonly used in the treatment of RB; however, their efficacy is often compromised by drug resistance and severe toxicity. The present study aimed to investigate and compare the toxicity and antitumor activity of the third‑generation platinum drugs, carboplatin and lobaplatin, in vitro and in vivo. The Y79 RB cell line was treated with carboplatin or lobaplatin in vitro and then used to establish xenografts in immunodeficient nude mice in vivo; the effects of pharmacological doses of these drugs were then assessed. High concentrations of carboplatin and lobaplatin markedly inhibited Y79 RB cell proliferation in vitro. In addition, the lobaplatin group exhibited higher proportions of early‑stage apoptotic cells than the carboplatin group, while no significant differences in the proportions of cells in the S phase were observed between the 2 groups, as shown by flow cytometry. Significant changes in the E2F1/Cdc25a/Cdk2 pathway in the RB cells were detected by RNA‑seq following carboplatin or lobaplatin intervention. RT‑qPCR, immunofluorescence and immunohistochemical analyses in vivo and in vitro demonstrated that the trends of drug‑induced inhibition of tumor pathological changes may have been regulated through the E2F1/Cdc25a/Cdk2 pathway, and that lobaplatin was more effective than carboplatin in controlling tumors in vivo. On the whole, the findings of the present study demonstrate that lobaplatin is associated with lower cytotoxicity and exerts more prominent therapeutic effects than carboplatin on Y79 RB cells in vitro and in mice in vivo.
Keywords: lobaplatin; carboplatin; retinoblastoma; therapeutic effects.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
