Ototoxicity and Platinum Uptake Following Cyclic Administration of Platinum-Based Chemotherapeutic Agents
- PMID: 32583132
- PMCID: PMC7445222
- DOI: 10.1007/s10162-020-00759-y
Ototoxicity and Platinum Uptake Following Cyclic Administration of Platinum-Based Chemotherapeutic Agents
Abstract
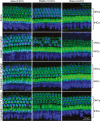
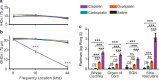
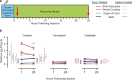
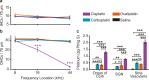
Cisplatin is a widely used anti-cancer drug used to treat a variety of cancer types. One of the side effects of this life-saving drug is irreversible ototoxicity, resulting in permanent hearing loss in many patients. In order to understand why cisplatin is particularly toxic to the inner ear, we compared the hearing loss and cochlear uptake of cisplatin to that of two related drugs, carboplatin and oxaliplatin. These three drugs are similar in that each contains a core platinum atom; however, carboplatin and oxaliplatin are considered less ototoxic than cisplatin. We delivered these three drugs to mice using a 6-week cyclic drug administration protocol. We performed the experiment twice, once using equimolar concentrations of the drugs and once using concentrations of the drugs more proportional to those used in the clinic. For both concentrations, we detected a significant hearing loss caused by cisplatin and no hearing loss caused by carboplatin or oxaliplatin. Cochlear uptake of each drug was measured using inductively coupled plasma mass spectrometry (ICP-MS) to detect platinum. Cochlear platinum levels were highest in mice treated with cisplatin followed by oxaliplatin, while carboplatin was largely excluded from the cochlea. Even when the drug doses were increased, cochlear platinum remained low in mice treated with oxaliplatin or carboplatin. We also examined drug clearance from the inner ear by measuring platinum levels at 1 h and 24 h after drug administration. Our findings suggest that the reduced cochlear platinum we observed with oxaliplatin and carboplatin were not due to increased clearance of these drugs relative to cisplatin. Taken together, our data indicate that the differential ototoxicity among cisplatin, carboplatin, and oxaliplatin is attributable to differences in cochlear uptake of these three drugs.
Keywords: ICP-MS; carboplatin; cisplatin; oxaliplatin.
Figures



Similar articles
-
The expression of copper transporters associated with the ototoxicity induced by platinum-based chemotherapeutic agents.Hear Res. 2020 Mar 15;388:107893. doi: 10.1016/j.heares.2020.107893. Epub 2020 Jan 17. Hear Res. 2020. PMID: 32006874
-
Cisplatin and oxaliplatin toxicity: importance of cochlear kinetics as a determinant for ototoxicity.J Natl Cancer Inst. 2009 Jan 7;101(1):37-47. doi: 10.1093/jnci/djn418. Epub 2008 Dec 30. J Natl Cancer Inst. 2009. PMID: 19116379 Free PMC article.
-
Ototoxicity among cisplatin, carboplatin, and oxaliplatin in zebrafish model.Environ Toxicol. 2024 Jul;39(7):4058-4065. doi: 10.1002/tox.24285. Epub 2024 Apr 25. Environ Toxicol. 2024. PMID: 38661261
-
Medical interventions for the prevention of platinum-induced hearing loss in children with cancer.Cochrane Database Syst Rev. 2019 May 7;5(5):CD009219. doi: 10.1002/14651858.CD009219.pub5. Cochrane Database Syst Rev. 2019. PMID: 31063591 Free PMC article.
-
Comparative adverse effect profiles of platinum drugs.Drug Saf. 1995 Oct;13(4):228-44. doi: 10.2165/00002018-199513040-00003. Drug Saf. 1995. PMID: 8573296 Review.
Cited by
-
Molecular Characteristics of Cisplatin-Induced Ototoxicity and Therapeutic Interventions.Int J Mol Sci. 2023 Nov 20;24(22):16545. doi: 10.3390/ijms242216545. Int J Mol Sci. 2023. PMID: 38003734 Free PMC article. Review.
-
Experimental animal models of drug-induced sensorineural hearing loss: a narrative review.Ann Transl Med. 2021 Sep;9(17):1393. doi: 10.21037/atm-21-2508. Ann Transl Med. 2021. PMID: 34733945 Free PMC article. Review.
-
Multiplex immunohistochemistry reveals cochlear macrophage heterogeneity and local auditory nerve inflammation in cisplatin-induced hearing loss.Front Neurol. 2022 Oct 20;13:1015014. doi: 10.3389/fneur.2022.1015014. eCollection 2022. Front Neurol. 2022. PMID: 36341090 Free PMC article.
-
Emerging and established therapies for chemotherapy-induced ototoxicity.J Cancer Surviv. 2023 Feb;17(1):17-26. doi: 10.1007/s11764-022-01317-6. Epub 2023 Jan 13. J Cancer Surviv. 2023. PMID: 36637631 Review.
-
Platinum drugs-related safety profile: The latest five-year analysis from FDA adverse event reporting system data.Front Oncol. 2023 Jan 11;12:1012093. doi: 10.3389/fonc.2022.1012093. eCollection 2022. Front Oncol. 2023. PMID: 36713566 Free PMC article.
References
-
- Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, Rethwisch V, Seipelt G, Homann N, Wilhelm G, Schuch G, Stoehlmacher J, Derigs HG, Hegewisch-Becker S, Grossmann J, Pauligk C, Atmaca A, Bokemeyer C, Knuth A, Jager E. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–1442. - PubMed
-
- Andersson A, Fagerberg J, Lewensohn R, Ehrsson H. Pharmacokinetics of cisplatin and its monohydrated complex in humans. J Pharm Sci. 1996;85:824–827. - PubMed
-
- Astolfi L, Simoni E, Ciorba A, Martini A. In vitro protective effects of Ginkgo bilboa against cisplatin toxicity in mouse cell line OCk3. Audiol Med. 2008;6(4):251–258.
-
- Bass JK, Huang J, Onar-Thomas A, Chang KW, Bhagat SP, Chintagumpala M, Bartels U, Gururangan S, Hassall T, Heath JA, McCowage G, Cohn RJ, Fisher MJ, Robinson G, Broniscer A, Gajjar A, Gurney JG. Concordance between the Chang and the International Society of Pediatric Oncology (SIOP) ototoxicity grading scales in patients treated with cisplatin for medulloblastoma. Pediatr Blood Cancer. 2014;61:601–605. - PMC - PubMed
-
- Becouarn Y, Agostini C, Trufflandier N, Boulanger V. Oxaliplatin: available data in non-colorectal gastrointestinal malignancies. Crit Rev Oncol Hematol. 2001;40:265–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

