Complement activation by human IgG antibodies to galactose-α-1,3-galactose
- PMID: 32583419
- PMCID: PMC7450175
- DOI: 10.1111/imm.13229
Complement activation by human IgG antibodies to galactose-α-1,3-galactose
Abstract
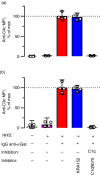
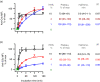
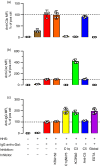
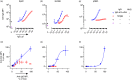
Some human antibodies may paradoxically inhibit complement activation on bacteria and enhance pathogen survival in humans. This property was also claimed for IgG antibodies reacting with terminal galactose-α-1,3-galactose (Galα3Gal; IgG anti-αGal), a naturally occurring and abundant antibody in human plasma that targets numerous different pathogens. To reinvestigate these effects, we used IgG anti-αGal affinity isolated from a pool of normal human IgG and human hypogammaglobulinaemia serum as a complement source. Flow cytometry was performed to examine antibody binding and complement deposition on pig erythrocytes, Escherichia coli O86 and Streptococcus pneumoniae serotype 9V. Specific nanobodies were used to block the effect of single complement factors and to delineate the complement pathways involved. IgG anti-αGal was capable of activating the classical complement pathway on all the tested target cells. The degree of activation was exponentially related to the density of bound antibody on E. coli O86 and pig erythrocytes, but more linearly on S. pneumoniae 9V. The alternative pathway of complement amplified complement deposition. Deposited C3 fragments covered the activating IgG anti-αGal, obstructing its detection and highlighting this as a likely general caveat in studies of antibody density and complement deposition. The inherent capacity for complement activation by the purified carbohydrate reactive IgG anti-αGal was similar to that of normal human IgG. We propose that the previously reported complement inhibition by IgG anti-αGal relates to suboptimal assay configurations, in contrast to the complement activating property of the antibodies demonstrated in this paper.
Keywords: alpha-galactosyl epitope; antibodies; antigens/peptides/epitopes; complement; human.
© 2020 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

