Bmi deficiency causes oxidative stress and intervertebral disc degeneration which can be alleviated by antioxidant treatment
- PMID: 32583517
- PMCID: PMC7417700
- DOI: 10.1111/jcmm.15528
Bmi deficiency causes oxidative stress and intervertebral disc degeneration which can be alleviated by antioxidant treatment
Abstract
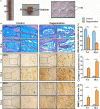
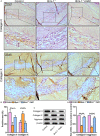
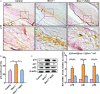
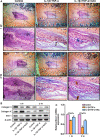
The transcriptional repressor Bmi-1 is involved in cell-cycle regulation and cell senescence, the deficiency of which has been shown to cause oxidative stress. This study investigated whether Bmi-1 deficiency plays a role in promoting disc degeneration and the effect of treatment with antioxidant N-acetylcysteine (NAC) on intervertebral disc degeneration. Bmi-1-/- mice were treated with the antioxidant NAC, supplied in drinking water (Bmi-1-/- +NAC). For in vitro experiments, mouse intervertebral discs were cultured under low oxygen tension and serum-limiting conditions in the presence of tumour necrosis factor α and interleukin 1β in order to mimic degenerative insult. Disc metabolism parameters in these in vitro and in vivo studies were evaluated by histopathological, immunohistochemical and molecular methods. Bmi-1-/- mice showed lower collagen Ⅱ and aggrecan levels and higher collagen Ⅹ levels than wild-type and Bmi-1-/- +NAC mice. Bmi-1-/- mice showed significantly lower superoxide dismutase (SOD)-1, SOD-2, glutathione peroxidase (GPX)-1 and GPX-3 levels than their wild-type littermates and Bmi-1-/- + NAC mice. Relative to Bmi-1-/- mice, the control and Bmi-1-/- +NAC mice showed significantly lower p16, p21, and p53 levels. These results demonstrate that Bmi-1 plays an important role in attenuating intervertebral disc degeneration in mice by inhibiting oxidative stress and cell apoptosis.
Keywords: Bmi-1; N-acetylcysteine; cell apoptosis; intervertebral disc degeneration; oxidative stress.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures

Similar articles
-
Effects of kartogenin on the attenuated nucleus pulposus cell degeneration of intervertebral discs induced by interleukin-1β and tumor necrosis factor-α.Int J Mol Med. 2018 Feb;41(2):749-756. doi: 10.3892/ijmm.2017.3283. Epub 2017 Nov 24. Int J Mol Med. 2018. PMID: 29207013 Free PMC article.
-
Targeting oxidative stress with amobarbital to prevent intervertebral disc degeneration: Part I. in vitro and ex vivo studies.Spine J. 2021 Jun;21(6):1021-1030. doi: 10.1016/j.spinee.2021.02.008. Epub 2021 Feb 19. Spine J. 2021. PMID: 33610806 Free PMC article.
-
Paraoxonase 1 Was Negatively Associated With Intervertebral Disc Degeneration.Spine (Phila Pa 1976). 2019 Sep;44(18):E1053-E1062. doi: 10.1097/BRS.0000000000003059. Spine (Phila Pa 1976). 2019. PMID: 30946296
-
The role of oxidative stress in intervertebral disc degeneration: Mechanisms and therapeutic implications.Ageing Res Rev. 2024 Jul;98:102323. doi: 10.1016/j.arr.2024.102323. Epub 2024 May 9. Ageing Res Rev. 2024. PMID: 38734147 Review.
-
[Effects of Oxidative Stress on Mitochondrial Functions and Intervertebral Disc Cells].Sichuan Da Xue Xue Bao Yi Xue Ban. 2024 Mar 20;55(2):249-255. doi: 10.12182/20240360201. Sichuan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38645848 Free PMC article. Review. Chinese.
Cited by
-
The causality between smoking and intervertebral disc degeneration mediated by IL-1β secreted by macrophage: A Mendelian randomization study.Heliyon. 2024 Aug 28;10(17):e37044. doi: 10.1016/j.heliyon.2024.e37044. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39286222 Free PMC article.
-
Cellular Senescence in Intervertebral Disc Aging and Degeneration: Molecular Mechanisms and Potential Therapeutic Opportunities.Biomolecules. 2023 Apr 18;13(4):686. doi: 10.3390/biom13040686. Biomolecules. 2023. PMID: 37189433 Free PMC article. Review.
-
Ipriflavone ameliorates intervertebral disc degeneration by inhibiting osteoporosis of vertebral body and pyroptosis of the nucleus pulposus in instability of lumbar spine and diabetic mice.Front Bioeng Biotechnol. 2025 Aug 5;13:1639117. doi: 10.3389/fbioe.2025.1639117. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40837020 Free PMC article.
-
Bmi1 regulate tooth and mandible development by inhibiting p16 signal pathway.J Cell Mol Med. 2021 May;25(9):4195-4203. doi: 10.1111/jcmm.16468. Epub 2021 Mar 21. J Cell Mol Med. 2021. PMID: 33745198 Free PMC article.
-
Oxidative stress in intervertebral disc degeneration: Molecular mechanisms, pathogenesis and treatment.Cell Prolif. 2023 Sep;56(9):e13448. doi: 10.1111/cpr.13448. Epub 2023 Mar 13. Cell Prolif. 2023. PMID: 36915968 Free PMC article. Review.
References
-
- Battié MC, Videman T, Levalahti E, Gill K, Kaprio J. Heritability of low back pain and the role of disc degeneration. Pain. 2007;131:272‐280. - PubMed
-
- Wang F, Cai F, Shi R, Wang XH, Wu XT. Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthritis Cartilage. 2016;24:398‐408. - PubMed
-
- Humzah M, Soames R. Human intervertebral disc: structure and function. Anat Rec. 1988;220:337‐356. - PubMed
-
- Sobajima S, Shimer AL, Chadderdon RC, et al. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real‐time polymerase chain reaction. Spine J. 2005;5:14‐23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

