Two-year follow-up of a randomized phase III clinical trial of nivolumab vs. the investigator's choice of therapy in the Asian population for recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141)
- PMID: 32583557
- PMCID: PMC7540331
- DOI: 10.1002/hed.26331
Two-year follow-up of a randomized phase III clinical trial of nivolumab vs. the investigator's choice of therapy in the Asian population for recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141)
Abstract
Background: The present study evaluated the 2-year survival of the Asian population in the CheckMate 141 trial.
Methods: The CheckMate 141 trial included patients with recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN). In the present study, 34 Asian patients (nivolumab group: 23 patients; investigator's choice of therapy [IC] group: 11 patients) were analyzed.
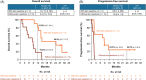
Results: The median overall survival (OS) was 12.1 and 6.2 months for the nivolumab and IC groups, respectively. The estimated 2-year OS rates were 22.7% and 0% for the nivolumab and IC groups, respectively. In the nivolumab group, the patients with any treatment-related adverse events (TRAEs), including skin-related disorders, showed better OS than the patients without any TRAEs.
Conclusions: Nivolumab demonstrated prolonged OS benefits in the Asian population with platinum-refractory R/M SCCHN and a favorable safety profile. TRAEs, including skin-related disorders, may be favorable prognostic factors for nivolumab efficacy.
Clinical trial registration: NCT02105636.
Keywords: Asian population; clinical trial; immunotherapy; nivolumab; squamous cell carcinoma of the head and neck.
© 2020 The Authors. Head & Neck published by Wiley Periodicals LLC.
Conflict of interest statement
Dr Yen reports grants from ONO Pharmaceutical and Bristol‐Myers Squibb. Dr Kiyota reports grants from ONO Pharmaceutical, AstraZeneca, Pfizer, and Chugai Pharmaceutical; honoraria from ONO Pharmaceutical, Bristol‐Myers Squibb, Merck Serono, Eisai, and Bayer. Dr Hanai reports grants from ONO Pharmaceutical and Bristol‐Myers Squibb. Dr Takahashi reports grants from ONO Pharmaceutical and Bristol‐Myers Squibb. Dr Yokota reports grants from ONO Pharmaceutical, Bristol‐Myers Squibb, AstraZeneca, Merck Biopharma, MSD, Novartis, Incyte, Eli Lilly, Eisai, and Chugai Pharmaceutical. Dr Iwae reports grants from ONO Pharmaceutical and Bristol‐Myers Squibb. Dr Shimizu reports grants from ONO Pharmaceutical and Bristol‐Myers Squibb. Dr Hong reports grants from ONO Pharmaceutical and Bristol‐Myers Squibb. Dr Goto reports grants from Bristol‐Myers Squibb; and grants, personal fees, and nonfinancial support from ONO Pharmaceutical; and grants, personal fees, and nonfinancial support from Takeda, Chugai, Kyowa Hakko Kirin, Taiho, and Mochida; personal fees and nonfinancial support from Yakult Honsha, Novartis, Bayer Yakuhin; and grants from Nippon Kayaku outside the submitted work. Dr Kang reports grants from ONO Pharmaceutical, AstraZeneca, and Eli Lilly. Dr Li reports grants from ONO Pharmaceutical and Bristol‐Myers Squibb. Dr Ferris reports grants and clinical trial support from AstraZeneca/MedImmune, Bristol‐Myers Squibb, Merck, Tesaro, and VentiRx Pharmaceuticals; advisory board/consultation fees from Amgen, AstraZeneca/MedImmune, Bain Capital Life Sciences, Bristol‐Myers Squibb, EMD Serono, GlaxoSmithKline, Iovance Biotherapeutics, Inc., Lilly, Merck, Numab Therapeutics AG, Oncorus, Inc., ONO Pharmaceutical Co. Ltd., Pfizer, PPD (Benitec, Immunicum), Regeneron Pharmaceuticals, Inc., Tesaro, Torque Therapeutics Inc., and TTMS. Dr Gillison reports grants from AstraZeneca, Bristol‐Myers Squibb, Kyowa, and Merck and personal fees from Amgen, AstraZeneca, Bristol‐Myers Squibb, Celgene, GlaxoSmithKline, Lilly, and Merck. Dr Tahara reports grants from ONO Pharmaceutical, Bristol‐Myers Squibb, Eisai, Otsuka, Boehringer Ingelheim, AstraZeneca, Pfizer, Novartis, and NanoCarrier and has received personal fees from ONO Pharmaceutical, Bristol‐Myers Squibb, Merck Sharp & Dohme, Bayer, Eisai, Otsuka, AstraZeneca, and Pfizer. T. Endo is an employee of ONO Pharmaceutical. V. Jayaprakash is an employee of Bristol‐Myers Squibb.
Figures


References
-
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;15:1941‐1953. - PubMed
-
- Katanoda K, Hori M, Matsuda T, et al. An updated report on the trends in cancer incidence and mortality in Japan, 1958‐2013. Jpn J Clin Oncol. 2015;45:390‐401. - PubMed
-
- Hwang TZ, Hsiao JR, Tsai CR, Chang JS. Incidence trends of human papillomavirus‐related head and neck cancer in Taiwan, 1995–2009. Int J Cancer. 2015;137:395‐408. - PubMed
-
- Choi SW, Thomson P. Increasing incidence of oral cancer in Hong Kong—who, where…and why? J Oral Pathol Med. 2019;48:483‐490. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

