The efficacy and safety of neoadjuvant chemotherapy on patients with advanced gastric cancer: A multicenter randomized clinical trial
- PMID: 32583567
- PMCID: PMC7433829
- DOI: 10.1002/cam4.3224
The efficacy and safety of neoadjuvant chemotherapy on patients with advanced gastric cancer: A multicenter randomized clinical trial
Abstract
Objectives: Exploring the efficacy and safety of perioperative chemotherapy on patients with AGC at different clinical and pathological stages.
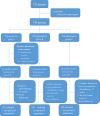
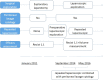
Methods: A phase III randomized, multicenter, trial comparing adjuvant (arm A) or perioperative S-1 plus oxaliplatin (SOX, arm B), and perioperative capecitabine plus oxaliplatin (XELOX, arm C) was initiated in T3/4, node + gastric cancer patients (unclear). Each patient received an 8-cycle chemotherapy (3 weeks for one cycle). Group arms B and C received two cycles preoperatively, and six cycles postoperatively. Primary endpoints were R0 resection rate and DFS, and secondary endpoints included OS, ORR, DCR, and safety. This study was registered on Clinicaltrials.gov. NCT01516944.
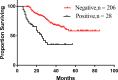
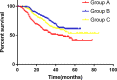
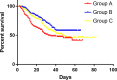
Results: A total of 749 patients were randomly assigned into groups A, B, and C. Group A received 1460 circles chemotherapy and group B received 1177 circles while group C received 1200 circles. R0 resection rates in the three groups were 81.7%, 88.7%, and 83.1%, respectively. The difference between groups A and B was considered to be statistically significant (P = .018), and no significant difference between groups B and C (P = .051). Hazard ratio were compared between groups B and C and DFS showed 0.72 (0.67-0.77 with 95% CI), Pnon-inferiority < .0001, Plog-rank = .064). The CI top limit actually lower than the estimated value of 1.38, which indicated noninferiority of SOX to XELOX.
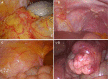
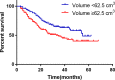
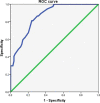
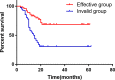
Conclusions: Compared with PAC, perioperative chemotherapy showed a significant improvement in R0 resection rates and prognosis in AGC patients with higher safety rates. This study was powered to show superiority of perioperative over adjuvant SOX, and noninferiority of SOX to XELOX. Volume measurement, repeated laparoscopic exploration combined with exfoliative cytology can be used as a supplementary method in the clinical staging and efficacy evaluation of AGC.
Keywords: SOX; XELOX; gastric cancer; neoadjuvant chemotherapy; survival analysis.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial.Lancet Oncol. 2021 Aug;22(8):1081-1092. doi: 10.1016/S1470-2045(21)00297-7. Epub 2021 Jul 9. Lancet Oncol. 2021. PMID: 34252374 Clinical Trial.
-
[Safety and effectiveness of oxaliplatin combined with capecitabine or oxaliplatin combined with S-1 neoadjuvant chemotherapy in the treatment of advanced gastric cancer].Zhonghua Wei Chang Wai Ke Za Zhi. 2021 Feb 25;24(2):138-144. doi: 10.3760/cma.j.cn.441530-20200721-00433. Zhonghua Wei Chang Wai Ke Za Zhi. 2021. PMID: 33508919 Chinese.
-
The protocol of a prospective, multicenter, randomized, controlled phase III study evaluating different cycles of oxaliplatin combined with S-1 (SOX) as neoadjuvant chemotherapy for patients with locally advanced gastric cancer: RESONANCE-II trial.BMC Cancer. 2021 Jan 5;21(1):20. doi: 10.1186/s12885-020-07764-7. BMC Cancer. 2021. PMID: 33402102 Free PMC article.
-
Comparison of S-1-based vs. capecitabine-based adjuvant chemotherapy for patients with gastric cancer: a systematic review and meta-analysis.Eur J Clin Pharmacol. 2021 Dec;77(12):1791-1804. doi: 10.1007/s00228-021-03187-w. Epub 2021 Jul 17. Eur J Clin Pharmacol. 2021. PMID: 34275019
-
Meta-analysis supporting noninferiority of oxaliplatin plus S-1 to cisplatin plus S-1 in first-line treatment of advanced gastric cancer (G-SOX study): indirect comparison with S-1 alone.Int J Clin Oncol. 2016 Aug;21(4):668-675. doi: 10.1007/s10147-015-0938-9. Epub 2016 Jan 5. Int J Clin Oncol. 2016. PMID: 26733020 Review.
Cited by
-
Safety and efficacy of robotic-assisted versus laparoscopic distal gastrectomy after neoadjuvant chemotherapy for advanced gastric cancer.Surg Endosc. 2023 Sep;37(9):6761-6770. doi: 10.1007/s00464-023-10122-w. Epub 2023 May 23. Surg Endosc. 2023. PMID: 37221415
-
Efficacy and safety of sintilimab plus XELOX as a neoadjuvant regimen in patients with locally advanced gastric cancer: A single-arm, open-label, phase II trial.Front Oncol. 2022 Aug 26;12:927781. doi: 10.3389/fonc.2022.927781. eCollection 2022. Front Oncol. 2022. PMID: 36091139 Free PMC article.
-
Combined systemic immune-inflammatory index (SII) and prognostic nutritional index (PNI) predicts chemotherapy response and prognosis in locally advanced gastric cancer patients receiving neoadjuvant chemotherapy with PD-1 antibody sintilimab and XELOX: a prospective study.BMC Gastroenterol. 2022 Mar 14;22(1):121. doi: 10.1186/s12876-022-02199-9. BMC Gastroenterol. 2022. PMID: 35287591 Free PMC article.
-
Short-Term Clinical Efficacy of Neoadjuvant Chemotherapy Combined With Laparoscopic Gastrectomy for Locally Advanced Siewert Type II and III Adenocarcinoma of the Esophagogastric Junction: A Retrospective, Propensity Score-Matched Study.Front Oncol. 2021 Sep 29;11:690662. doi: 10.3389/fonc.2021.690662. eCollection 2021. Front Oncol. 2021. PMID: 34660265 Free PMC article.
-
Multidisciplinary treatment for locally advanced gastric cancer: A systematic review and network meta-analysis.J Minim Access Surg. 2023 Jul-Sep;19(3):335-347. doi: 10.4103/jmas.jmas_170_22. J Minim Access Surg. 2023. PMID: 37282430 Free PMC article. Review.
References
-
- International Agency for Research on Cancer (IARC) . GLOBOCAN 2018: estimated cancer incidence, mortality and prevalence worldwide in 2018; 2018.
-
- Yamashita K, Kurokawa Y, Yamamoto K, et al. Risk factors for poor compliance with adjuvant S‐1 chemotherapy for gastric cancer: a multicenter retrospective study. Ann Surg Oncol. 2017;24:2639‐2645. - PubMed
-
- Bang Y‐J, Kim Y‐W, Yang H‐K, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open‐label, randomised controlled trial. Lancet. 2012;379:315‐321. - PubMed
-
- Sasako M, Sakuramoto S, Katai H, et al. Five‐year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S‐1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387‐4393. - PubMed
-
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11‐20. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

