The efficacy and safety of neoadjuvant chemotherapy on patients with advanced gastric cancer: A multicenter randomized clinical trial
- PMID: 32583567
- PMCID: PMC7433829
- DOI: 10.1002/cam4.3224
The efficacy and safety of neoadjuvant chemotherapy on patients with advanced gastric cancer: A multicenter randomized clinical trial
Abstract
Objectives: Exploring the efficacy and safety of perioperative chemotherapy on patients with AGC at different clinical and pathological stages.
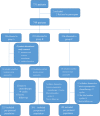
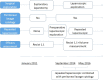
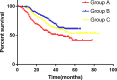
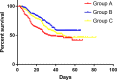
Methods: A phase III randomized, multicenter, trial comparing adjuvant (arm A) or perioperative S-1 plus oxaliplatin (SOX, arm B), and perioperative capecitabine plus oxaliplatin (XELOX, arm C) was initiated in T3/4, node + gastric cancer patients (unclear). Each patient received an 8-cycle chemotherapy (3 weeks for one cycle). Group arms B and C received two cycles preoperatively, and six cycles postoperatively. Primary endpoints were R0 resection rate and DFS, and secondary endpoints included OS, ORR, DCR, and safety. This study was registered on Clinicaltrials.gov. NCT01516944.
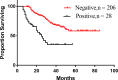
Results: A total of 749 patients were randomly assigned into groups A, B, and C. Group A received 1460 circles chemotherapy and group B received 1177 circles while group C received 1200 circles. R0 resection rates in the three groups were 81.7%, 88.7%, and 83.1%, respectively. The difference between groups A and B was considered to be statistically significant (P = .018), and no significant difference between groups B and C (P = .051). Hazard ratio were compared between groups B and C and DFS showed 0.72 (0.67-0.77 with 95% CI), Pnon-inferiority < .0001, Plog-rank = .064). The CI top limit actually lower than the estimated value of 1.38, which indicated noninferiority of SOX to XELOX.
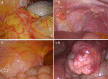
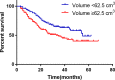
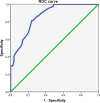
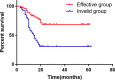
Conclusions: Compared with PAC, perioperative chemotherapy showed a significant improvement in R0 resection rates and prognosis in AGC patients with higher safety rates. This study was powered to show superiority of perioperative over adjuvant SOX, and noninferiority of SOX to XELOX. Volume measurement, repeated laparoscopic exploration combined with exfoliative cytology can be used as a supplementary method in the clinical staging and efficacy evaluation of AGC.
Keywords: SOX; XELOX; gastric cancer; neoadjuvant chemotherapy; survival analysis.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- International Agency for Research on Cancer (IARC) . GLOBOCAN 2018: estimated cancer incidence, mortality and prevalence worldwide in 2018; 2018.
-
- Yamashita K, Kurokawa Y, Yamamoto K, et al. Risk factors for poor compliance with adjuvant S‐1 chemotherapy for gastric cancer: a multicenter retrospective study. Ann Surg Oncol. 2017;24:2639‐2645. - PubMed
-
- Bang Y‐J, Kim Y‐W, Yang H‐K, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open‐label, randomised controlled trial. Lancet. 2012;379:315‐321. - PubMed
-
- Sasako M, Sakuramoto S, Katai H, et al. Five‐year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S‐1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387‐4393. - PubMed
-
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11‐20. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

