Lysosomal dysfunction-induced autophagic stress in diabetic kidney disease
- PMID: 32583573
- PMCID: PMC7412686
- DOI: 10.1111/jcmm.15301
Lysosomal dysfunction-induced autophagic stress in diabetic kidney disease
Abstract
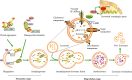
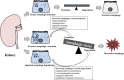
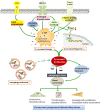
The catabolic process that delivers cytoplasmic constituents to the lysosome for degradation, known as autophagy, is thought to act as a cytoprotective mechanism in response to stress or as a pathogenic process contributing towards cell death. Animal and human studies have shown that autophagy is substantially dysregulated in renal cells in diabetes, suggesting that activating autophagy could be a therapeutic intervention. However, under prolonged hyperglycaemia with impaired lysosome function, increased autophagy induction that exceeds the degradative capacity in cells could contribute toward autophagic stress or even the stagnation of autophagy, leading to renal cytotoxicity. Since lysosomal function is likely key to linking the dual cytoprotective and cytotoxic actions of autophagy, it is important to develop novel pharmacological agents that improve lysosomal function and restore autophagic flux. In this review, we first provide an overview of the autophagic-lysosomal pathway, particularly focusing on stages of lysosomal degradation during autophagy. Then, we discuss the role of adaptive autophagy and autophagic stress based on lysosomal function. More importantly, we focus on the role of autophagic stress induced by lysosomal dysfunction according to the pathogenic factors (including high glucose, advanced glycation end products (AGEs), urinary protein, excessive reactive oxygen species (ROS) and lipid overload) in diabetic kidney disease (DKD), respectively. Finally, therapeutic possibilities aimed at lysosomal restoration in DKD are introduced.
Keywords: autophagic stress; autophagy; diabetic kidney disease; lysosomal dysfunction.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures
References
-
- Zhang L, Long J, Jiang W, et al. Trends in chronic kidney disease in China. N Engl J Med. 2016;375:905‐906. - PubMed
-
- Jefferson JA, Shankland SJ, Pichler RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 2008;74:22‐36. - PubMed
-
- Warren AM, Knudsen ST, Cooper ME. Diabetic nephropathy: an insight into molecular mechanisms and emerging therapies. Expert Opin Ther Targets. 2019;23:579‐591. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

