SCYL1 arginine methylation by PRMT1 is essential for neurite outgrowth via Golgi morphogenesis
- PMID: 32583741
- PMCID: PMC7543066
- DOI: 10.1091/mbc.E20-02-0100
SCYL1 arginine methylation by PRMT1 is essential for neurite outgrowth via Golgi morphogenesis
Abstract
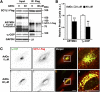
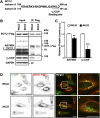
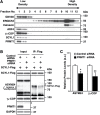
Arginine methylation is a common posttranslational modification that modulates protein function. SCY1-like pseudokinase 1 (SCYL1) is crucial for neuronal functions and interacts with γ2-COP to form coat protein complex I (COPI) vesicles that regulate Golgi morphology. However, the molecular mechanism by which SCYL1 is regulated remains unclear. Here, we report that the γ2-COP-binding site of SCYL1 is arginine-methylated by protein arginine methyltransferase 1 (PRMT1) and that SCYL1 arginine methylation is important for the interaction of SCYL1 with γ2-COP. PRMT1 was colocalized with SCYL1 in the Golgi fraction. Inhibition of PRMT1 suppressed axon outgrowth and dendrite complexity via abnormal Golgi morphology. Knockdown of SCYL1 by small interfering RNA (siRNA) inhibited axon outgrowth, and the inhibitory effect was rescued by siRNA-resistant SCYL1, but not SCYL1 mutant, in which the arginine methylation site was replaced. Thus, PRMT1 regulates Golgi morphogenesis via SCYL1 arginine methylation. We propose that SCYL1 arginine methylation by PRMT1 contributes to axon and dendrite morphogenesis in neurons.
Figures






References
-
- Bartel RL, Borchardt RT. (1984). Effects of adenosine dialdehyde on S-adenosylhomocysteine hydrolase and S-adenosylmethionine-dependent transmethylations in mouse L929 cells. Mol Pharmacol , 418–424. - PubMed
-
- Burman JL, Bourbonniere L, Philie J, Stroh T, Dejgaard SY, Presley JF, McPherson PS. (2008). Scyl1, mutated in a recessive form of spinocerebellar neurodegeneration, regulates COPI-mediated retrograde traffic. J Biol Chem , 22774–22786. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

