Immunology of COVID-19: Mechanisms, clinical outcome, diagnostics, and perspectives-A report of the European Academy of Allergy and Clinical Immunology (EAACI)
- PMID: 32584441
- PMCID: PMC7361752
- DOI: 10.1111/all.14462
Immunology of COVID-19: Mechanisms, clinical outcome, diagnostics, and perspectives-A report of the European Academy of Allergy and Clinical Immunology (EAACI)
Abstract
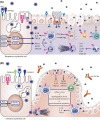
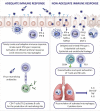
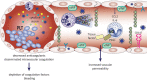
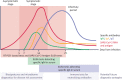
With the worldwide spread of the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) resulting in declaration of a pandemic by the World Health Organization (WHO) on March 11, 2020, the SARS-CoV-2-induced coronavirus disease-19 (COVID-19) has become one of the main challenges of our times. The high infection rate and the severe disease course led to major safety and social restriction measures worldwide. There is an urgent need of unbiased expert knowledge guiding the development of efficient treatment and prevention strategies. This report summarizes current immunological data on mechanisms associated with the SARS-CoV-2 infection and COVID-19 development and progression to the most severe forms. We characterize the differences between adequate innate and adaptive immune response in mild disease and the deep immune dysfunction in the severe multiorgan disease. The similarities of the human immune response to SARS-CoV-2 and the SARS-CoV and MERS-CoV are underlined. We also summarize known and potential SARS-CoV-2 receptors on epithelial barriers, immune cells, endothelium and clinically involved organs such as lung, gut, kidney, cardiovascular, and neuronal system. Finally, we discuss the known and potential mechanisms underlying the involvement of comorbidities, gender, and age in development of COVID-19. Consequently, we highlight the knowledge gaps and urgent research requirements to provide a quick roadmap for ongoing and needed COVID-19 studies.
Keywords: COVID-19 comorbidity; COVID-19 immunity; COVID-19 multimorbidity; COVID-19 prevention; COVID-19 treatment; SARS; SARS-CoV-2 receptors.
© 2020 EAACI and John Wiley and Sons A/S. Published by John Wiley and Sons Ltd.
Figures



References
-
- Li W, Shi Z, Yu M, et al. Bats are natural reservoirs of SARS‐like coronaviruses. Science. 2005;310(5748):676‐679. - PubMed

