Critical role of GRP receptor-expressing neurons in the spinal transmission of imiquimod-induced psoriatic itch
- PMID: 32584520
- PMCID: PMC7722649
- DOI: 10.1002/npr2.12120
Critical role of GRP receptor-expressing neurons in the spinal transmission of imiquimod-induced psoriatic itch
Abstract
Aim: Ample evidence indicates that gastrin-releasing peptide receptor (GRPR)-expressing neurons play a critical role in the transmission of acute itch. However, the pathophysiology of spinal mechanisms underlying intractable itch such as psoriasis remains unclear. In this study, we aimed to determine whether itch-responsive GRPR+ neurons contribute to the spinal transmission of imiquimod (IMQ)-induced psoriatic itch.
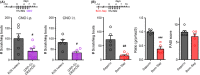
Methods: To generate a psoriasis model, C57BL/6J mice received a daily topical application of 5% IMQ cream on their shaved back skin for 7-10 consecutive days. GRP+ neurons were inhibited using Cre-dependent expression of Gi-designer receptors exclusively activated by designer drugs (DREADDs), while GRPR+ neurons were ablated by intrathecal administration of bombesin-saporin.
Results: Repeated topical application of IMQ elicited psoriasis-like dermatitis and scratching behaviors. The mRNA expression levels of GRP and GRPR were upregulated in the cervical spinal dorsal horn (SDH) on days 7 and 10 after IMQ application. Either chemogenetic silencing of GRP+ neurons by Gi-DREADD or ablation of GRPR+ neurons significantly attenuated IMQ-induced scratching behaviors.
Conclusion: The GRP-GRPR system might be enhanced in the SDH, and itch-responsive GRPR+ neurons largely contribute to intractable itch in a mouse model of psoriasis.
Keywords: AMPA; GRP; pruritus; psoriasis; spinal cord.
© 2020 The Authors. Neuropsychopharmacology Reports published by John Wiley & Sons Australia, Ltd on behalf of the Japanese Society of NeuropsychoPharmacology.
Conflict of interest statement
The authors have no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

