Molecular Dynamics and Physical Stability of Ibuprofen in Binary Mixtures with an Acetylated Derivative of Maltose
- PMID: 32584584
- PMCID: PMC7467776
- DOI: 10.1021/acs.molpharmaceut.0c00517
Molecular Dynamics and Physical Stability of Ibuprofen in Binary Mixtures with an Acetylated Derivative of Maltose
Abstract
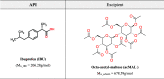
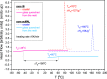
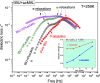
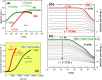
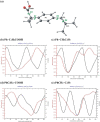
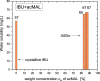
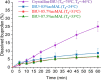
In this paper, we explore the strategy increasingly used to improve the bioavailability of poorly water-soluble crystalline drugs by formulating their amorphous solid dispersions. We focus on the potential application of a low molecular weight excipient octaacetyl-maltose (acMAL) to prepare physically stable amorphous solid dispersions with ibuprofen (IBU) aimed at enhancing water solubility of the drug compared to that of its crystalline counterpart. We thoroughly investigate global and local molecular dynamics, thermal properties, and physical stability of the IBU+acMAL binary systems by using broadband dielectric spectroscopy and differential scanning calorimetry as well as test their water solubility and dissolution rate. The obtained results are extensively discussed by analyzing several factors considered to affect the physical stability of amorphous systems, including those related to the global mobility, such as plasticization/antiplasticization effects, the activation energy, fragility parameter, and the number of dynamically correlated molecules as well as specific intermolecular interactions like hydrogen bonds, supporting the latter by density functional theory calculations. The observations made for the IBU+acMAL binary systems and drawn recommendations give a better insight into our understanding of molecular mechanisms governing the physical stability of amorphous solid dispersions.
Keywords: amorphous drug; amorphous solid dispersion; crystallization; devitrification; glass transition; ibuprofen; molecular dynamics; physical stability.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Patel R.; Patel N.; Patel N. M. A novel approach for dissolution enhancement of Ibuprofen by preparing floating granules. Int. J. Res. Pharm. Sci. 2010, 1, 57–64.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

