Carotid body chemosensitivity at 1.6 ATA breathing air versus 100% oxygen
- PMID: 32584669
- PMCID: PMC7473945
- DOI: 10.1152/japplphysiol.00275.2020
Carotid body chemosensitivity at 1.6 ATA breathing air versus 100% oxygen
Abstract
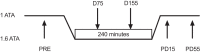
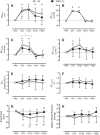
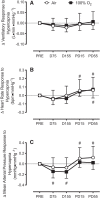
Hyperoxia reduces the ventilatory response to hypercapnia by suppressing carotid body (CB) activation. This effect may contribute to CO2 retention during underwater diving due to the high arterial O2 content associated with hyperbaria. We tested the hypothesis that CB chemosensitivity to hypercapnia and hypoxia is attenuated during hyperbaria. Ten subjects completed two, 4-h dry dives at 1.6 atmosphere absolute (ATA) breathing either 21% O2 (Air) or 100% O2 (100% O2). CB chemosensitivity was assessed using brief hypercapnic ventilatory response ([Formula: see text]) and hypoxic ventilatory response ([Formula: see text]) tests predive, 75 and 155 min into the dives, and 15 and 55 min postdive. End-tidal CO2 pressure increased during the dive at 75 and 155 min [Air: +9 (SD 4) mmHg and +8 (SD 4) mmHg versus 100% O2: +6 (SD 4) mmHg and +5 (SD 3) mmHg; all P < 0.01] and was higher while breathing Air (P < 0.01). [Formula: see text] was unchanged during the dive (P = 0.73) and was not different between conditions (P = 0.47). However, [Formula: see text] was attenuated from predive during the dive at 155 min breathing Air [-0.035 (SD 0.037) L·min·mmHg-1; P = 0.02] and at both time points while breathing 100% O2 [-0.035 (SD 0.052) L·min·mmHg-1 and -0.034 (SD 0.064) L·min·mmHg-1; P = 0.02 and P = 0.02, respectively]. These data indicate that the CB chemoreceptors do not appear to contribute to CO2 retention in hyperbaria.NEW & NOTEWORTHY We demonstrate that carotid body chemosensitivity to brief exposures of hypercapnia was unchanged during a 4-h dive in a dry hyperbaric chamber at 1.6 ATA regardless of breathing gas condition [i.e., air (21% O2) versus 100% oxygen]. Therefore, it appears that an attenuation of carotid body chemosensitivity to hypercapnia does not contribute to CO2 retention in hyperbaria.
Keywords: CO2 retention; carotid body chemosensitivity; hyperbaria; hyperoxia.
Conflict of interest statement
B. M. Clemency is a speaker and consultant for Stryker Corporation. No conflicts of interest, financial or otherwise, are declared by the other authors.
Figures


References
-
- Arieli R, Arieli Y, Daskalovic Y, Eynan M, Abramovich A. CNS oxygen toxicity in closed-circuit diving: signs and symptoms before loss of consciousness. Aviat Space Environ Med 77: 1153–1157, 2006. - PubMed
-
- Arieli R, Shochat T, Adir Y. CNS toxicity in closed-circuit oxygen diving: symptoms reported from 2527 dives. Aviat Space Environ Med 77: 526–532, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

