Remifentanil preconditioning protects against hypoxia-induced senescence and necroptosis in human cardiac myocytes in vitro
- PMID: 32584786
- PMCID: PMC7425462
- DOI: 10.18632/aging.103604
Remifentanil preconditioning protects against hypoxia-induced senescence and necroptosis in human cardiac myocytes in vitro
Abstract
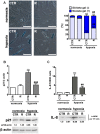
Remifentanil and other opioids are suggested to be protective against ischemia-reperfusion injury in animal models and coronary artery bypass surgery patients, however the molecular basis of such protection is far from being understood. In the present study, we have used a model of human cardiomyocytes treated with the hypoxia-mimetic agent cobalt chloride to investigate remifentanil preconditioning-based adaptive responses and underlying mechanisms. Hypoxic conditions promoted oxidative and nitrosative stress, p21-mediated cellular senescence and the activation of necroptotic pathway that was accompanied by a 2.2-, 9.6- and 8.2-fold increase in phosphorylation status of mixed lineage kinase domain-like pseudokinase (MLKL) and release of pro-inflammatory cytokine IL-8 and cardiac troponin I, a marker of myocardial damage, respectively. Remifentanil preconditioning was able to lower hypoxia-mediated protein carbonylation and limit MLKL-based signaling and pro-inflammatory response to almost normoxic control levels, and decrease hypoxia-induced pro-senescent activity of about 21% compared to control hypoxic conditions. In summary, we have shown for the first time that remifentanil can protect human cardiomyocytes against hypoxia-induced cellular senescence and necroptosis that may have importance with respect to the use of remifentanil to diminish myocardial ischemia and reperfusion injury in patients undergoing cardiac surgery.
Keywords: cardiomyocytes; hypoxia; necroptosis; remifentanil; senescence.
Conflict of interest statement
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

