12(S)-HETE mediates diabetes-induced endothelial dysfunction by activating intracellular endothelial cell TRPV1
- PMID: 32584793
- PMCID: PMC7456227
- DOI: 10.1172/JCI136621
12(S)-HETE mediates diabetes-induced endothelial dysfunction by activating intracellular endothelial cell TRPV1
Abstract
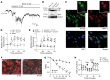
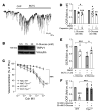
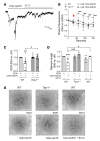
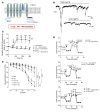
Patients with diabetes develop endothelial dysfunction shortly after diabetes onset that progresses to vascular disease underlying the majority of diabetes-associated comorbidities. Increased lipid peroxidation, mitochondrial calcium overload, and mitochondrial dysfunction are characteristics of dysfunctional endothelial cells in diabetic patients. We here identified that targeting the lipid peroxidation product 12(S)-hydroxyeicosatetraenoic acid-induced [12(S)-HETE-induced] activation of the intracellularly located cation channel transient receptor potential vanilloid 1 (TRPV1) in endothelial cells is a means to causally control early-stage vascular disease in type I diabetic mice. Mice with an inducible, endothelium-specific 12/15-lipoxygenase (12/15Lo) knockout were protected similarly to TRPV1-knockout mice from type 1 diabetes-induced endothelial dysfunction and impaired vascular regeneration following arterial injury. Both 12(S)-HETE in concentrations found in diabetic patients and TRPV1 agonists triggered mitochondrial calcium influx and mitochondrial dysfunction in endothelial cells, and 12(S)-HETE effects were absent in endothelial cells from TRPV1-knockout mice. As a therapeutic consequence, we found that a peptide targeting 12(S)-HETE-induced TRPV1 interaction at the TRPV1 TRP box ameliorated diabetes-induced endothelial dysfunction and augmented vascular regeneration in diabetic mice. Our findings suggest that pharmacological targeting of increased endothelial lipid peroxidation can attenuate diabetes-induced comorbidities related to vascular disease.
Keywords: Diabetes; Eicosanoids; Vascular Biology; endothelial cells.
Conflict of interest statement
Figures

Similar articles
-
Development of heart failure with preserved ejection fraction in type 2 diabetic mice is ameliorated by preserving vascular function.Life Sci. 2021 Nov 1;284:119925. doi: 10.1016/j.lfs.2021.119925. Epub 2021 Sep 1. Life Sci. 2021. PMID: 34480933 Free PMC article.
-
TRPV1-mediated UCP2 upregulation ameliorates hyperglycemia-induced endothelial dysfunction.Cardiovasc Diabetol. 2013 Apr 22;12:69. doi: 10.1186/1475-2840-12-69. Cardiovasc Diabetol. 2013. PMID: 23607427 Free PMC article.
-
Endothelin-mediated in vivo pressor responses following TRPV1 activation.Am J Physiol Heart Circ Physiol. 2011 Sep;301(3):H1135-42. doi: 10.1152/ajpheart.00082.2011. Epub 2011 Jun 24. Am J Physiol Heart Circ Physiol. 2011. PMID: 21705674
-
The contribution of TRPV1 channel to 20-HETE-Aggravated ischemic neuronal injury.Prostaglandins Other Lipid Mediat. 2018 Jul;137:63-68. doi: 10.1016/j.prostaglandins.2018.07.001. Epub 2018 Jul 6. Prostaglandins Other Lipid Mediat. 2018. PMID: 30041768 Free PMC article. Review.
-
Vascular complications in diabetes mellitus: the role of endothelial dysfunction.Clin Sci (Lond). 2005 Aug;109(2):143-59. doi: 10.1042/CS20050025. Clin Sci (Lond). 2005. PMID: 16033329 Review.
Cited by
-
TRPV1 Channel: A Noxious Signal Transducer That Affects Mitochondrial Function.Int J Mol Sci. 2020 Nov 24;21(23):8882. doi: 10.3390/ijms21238882. Int J Mol Sci. 2020. PMID: 33255148 Free PMC article. Review.
-
Ca2+ Signaling in Cardiac Fibroblasts: An Emerging Signaling Pathway Driving Fibrotic Remodeling in Cardiac Disorders.Biomedicines. 2025 Mar 17;13(3):734. doi: 10.3390/biomedicines13030734. Biomedicines. 2025. PMID: 40149710 Free PMC article. Review.
-
Empagliflozin-Pretreated MSC-Derived Exosomes Enhance Angiogenesis and Wound Healing via PTEN/AKT/VEGF Pathway.Int J Nanomedicine. 2025 Apr 22;20:5119-5136. doi: 10.2147/IJN.S512074. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40297404 Free PMC article.
-
Anticancer Activity of Natural and Semi-Synthetic Drimane and Coloratane Sesquiterpenoids.Molecules. 2022 Apr 13;27(8):2501. doi: 10.3390/molecules27082501. Molecules. 2022. PMID: 35458699 Free PMC article. Review.
-
Consequences of COVID-19 on the cardiovascular and renal systems.Sleep Med. 2022 Dec;100:31-38. doi: 10.1016/j.sleep.2022.07.011. Epub 2022 Aug 3. Sleep Med. 2022. PMID: 35994936 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

