Co-localization and confinement of ecto-nucleotidases modulate extracellular adenosine nucleotide distributions
- PMID: 32584811
- PMCID: PMC7316229
- DOI: 10.1371/journal.pcbi.1007903
Co-localization and confinement of ecto-nucleotidases modulate extracellular adenosine nucleotide distributions
Abstract
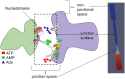
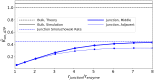
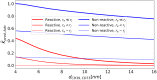
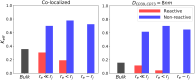
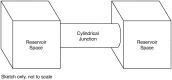
Nucleotides comprise small molecules that perform critical signaling roles in biological systems. Adenosine-based nucleotides, including adenosine tri-, di-, and mono-phosphate, are controlled through their rapid degradation by diphosphohydrolases and ecto-nucleotidases (NDAs). The interplay between nucleotide signaling and degradation is especially important in synapses formed between cells, which create signaling 'nanodomains'. Within these 'nanodomains', charged nucleotides interact with densely-packed membranes and biomolecules. While the contributions of electrostatic and steric interactions within such nanodomains are known to shape diffusion-limited reaction rates, less is understood about how these factors control the kinetics of nucleotidase activity. To quantify these factors, we utilized reaction-diffusion numerical simulations of 1) adenosine triphosphate (ATP) hydrolysis into adenosine monophosphate (AMP) and 2) AMP into adenosine (Ado) via two representative nucleotidases, CD39 and CD73. We evaluate these sequentially-coupled reactions in nanodomain geometries representative of extracellular synapses, within which we localize the nucleotidases. With this model, we find that 1) nucleotidase confinement reduces reaction rates relative to an open (bulk) system, 2) the rates of AMP and ADO formation are accelerated by restricting the diffusion of substrates away from the enzymes, and 3) nucleotidase co-localization and the presence of complementary (positive) charges to ATP enhance reaction rates, though the impact of these contributions on nucleotide pools depends on the degree to which the membrane competes for substrates. As a result, these contributions integratively control the relative concentrations and distributions of ATP and its metabolites within the junctional space. Altogether, our studies suggest that CD39 and CD73 nucleotidase activity within junctional spaces can exploit their confinement and favorable electrostatic interactions to finely control nucleotide signaling.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Crowding within synaptic junctions influences the degradation of nucleotides by CD39 and CD73 ectonucleotidases.Biophys J. 2022 Jan 18;121(2):309-318. doi: 10.1016/j.bpj.2021.12.013. Epub 2021 Dec 16. Biophys J. 2022. PMID: 34922916 Free PMC article.
-
Enzyme kinetics and pharmacological characterization of nucleotidases released from the guinea pig isolated vas deferens during nerve stimulation: evidence for a soluble ecto-nucleoside triphosphate diphosphohydrolase-like ATPase and a soluble ecto-5'-nucleotidase-like AMPase.J Pharmacol Exp Ther. 2002 Sep;302(3):992-1001. doi: 10.1124/jpet.102.033332. J Pharmacol Exp Ther. 2002. PMID: 12183656
-
Assessment of ATP metabolism to adenosine by ecto-nucleotidases carried by tumor-derived small extracellular vesicles.Purinergic Signal. 2025 Apr;21(2):339-352. doi: 10.1007/s11302-024-10038-7. Epub 2024 Jul 27. Purinergic Signal. 2025. PMID: 39066830 Free PMC article.
-
Ectonucleoside triphosphate diphosphohydrolases and ecto-5'-nucleotidase in purinergic signaling: how the field developed and where we are now.Purinergic Signal. 2021 Mar;17(1):117-125. doi: 10.1007/s11302-020-09755-6. Epub 2020 Dec 17. Purinergic Signal. 2021. PMID: 33336318 Free PMC article. Review.
-
Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities.Crit Rev Biochem Mol Biol. 2014 Nov-Dec;49(6):473-97. doi: 10.3109/10409238.2014.953627. Crit Rev Biochem Mol Biol. 2014. PMID: 25418535 Review.
Cited by
-
Crowding within synaptic junctions influences the degradation of nucleotides by CD39 and CD73 ectonucleotidases.Biophys J. 2022 Jan 18;121(2):309-318. doi: 10.1016/j.bpj.2021.12.013. Epub 2021 Dec 16. Biophys J. 2022. PMID: 34922916 Free PMC article.
-
Purinoreceptors and ectonucleotidases control ATP-induced calcium waveforms and calcium-dependent responses in microglia: Roles of P2 receptors and CD39 in ATP-stimulated microglia.Front Physiol. 2023 Jan 9;13:1037417. doi: 10.3389/fphys.2022.1037417. eCollection 2022. Front Physiol. 2023. PMID: 36699679 Free PMC article.
-
Mechanical Principles Governing the Shapes of Dendritic Spines.Front Physiol. 2021 Jun 16;12:657074. doi: 10.3389/fphys.2021.657074. eCollection 2021. Front Physiol. 2021. PMID: 34220531 Free PMC article.
-
Molecular Mechanics Study of Flow and Surface Influence in Ligand-Protein Association.Front Mol Biosci. 2021 May 10;8:659687. doi: 10.3389/fmolb.2021.659687. eCollection 2021. Front Mol Biosci. 2021. PMID: 34041265 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

