Biologic excipients: Importance of clinical awareness of inactive ingredients
- PMID: 32584876
- PMCID: PMC7316246
- DOI: 10.1371/journal.pone.0235076
Biologic excipients: Importance of clinical awareness of inactive ingredients
Abstract
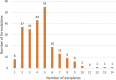
Due to the complexity and fragility of biological drug products, several challenges exist in their formulation development. Excipients are added to increase product stability, maintain tonicity, and facilitate drug delivery. The potential implications of these additive substances merit clinical consideration. We assessed the safety risk of excipients on the basis of their type and variability through an assessment framework, which quantifies excipient complexity in 230 biological formulations, and identifies excipient-related adverse events through published case reports. A biologic on average contained 4.45 excipients, half of that found in oral medications. The frequency distribution was heavily skewed towards the most commonly occurring excipients: water (40.4%), sodium chloride (38.3%), polysorbate 80 (28.7%), sucrose (24.4%), and mannitol (20.9%), with 44.4% of formulations not listing the concentration of the most commonly occurring inactive ingredients. A literature search revealed only 17 case reports of excipient-related adverse events, suggesting the need for more clarity for clinicians on the safety of chemical additives. These cases included injection site reactions, anaphylaxis, hyperglycemia, and acute renal failure. With the expansion of the biopharmaceutical market, it is important to consider the safety data of biologic excipients, so that therapy can be tailored appropriately for a specific patient.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- U.S. Food and Drug Administration. Inactive Ingredient Field Descriptions. Available from: https://www.fda.gov/drugs/informationondrugs/ucm075230.htm. Last updated 15 May 2015.
-
- U.S. Food and Drug Administration. Remarks from FDA Commissioner Scott Gottlieb, M.D., as prepared for delivery at the Brookings Institution on the release of the FDA’s Biosimilars Action Plan. July 2018.
-
- U.S. Food and Drug Administration. Biosimilars Action Plan: Balancing Innovation and Competition. July 2018. Available from: https://www.fda.gov/media/114574/download
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical