A Zika virus primary isolate induces neuroinflammation, compromises the blood-brain barrier and upregulates CXCL12 in adult macaques
- PMID: 32585067
- PMCID: PMC8018016
- DOI: 10.1111/bpa.12873
A Zika virus primary isolate induces neuroinflammation, compromises the blood-brain barrier and upregulates CXCL12 in adult macaques
Abstract
Zika virus (ZIKV) is a flavivirus that can cause neuropathogenesis in adults and fetal neurologic malformation following the infection of pregnant women. We used a nonhuman primate model, the Indian-origin Rhesus macaque (IRM), to gain insight into virus-associated hallmarks of ZIKV-induced adult neuropathology. We find that the virus causes prevalent acute and chronic neuroinflammation and chronic disruption of the blood-brain barrier (BBB) in adult animals. ZIKV infection resulted in specific short- and long-term augmented expression of the chemokine CXCL12 in the central nervous system (CNS)of adult IRMs. Moreover, CXCL12 expression persists long after the initial viral infection is apparently cleared. CXCL12 plays a key role both in regulating lymphocyte trafficking through the BBB to the CNS and in mediating repair of damaged neural tissue including remyelination. Understanding how CXCL12 expression is controlled will likely be of central importance in the definition of ZIKV-associated neuropathology in adults.
Keywords: CXCL12; Zika virus; blood-brain barrier; flavivirus; nonhuman primate.
© 2020 International Society of Neuropathology.
Conflict of interest statement
The authors have no financial or non‐financial competing interests.
Figures

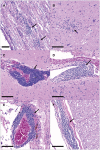
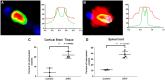

References
-
- Al‐Obaidi MMJ, Bahadoran A, Har LS, Mui WS, Rajarajeswaran J, Zandi K et al (2017) Japanese encephalitis virus disrupts blood‐brain barrier and modulates apoptosis proteins in THBMEC cells. Virus Res 233:17–28. - PubMed
-
- Araujo AQ, Silva MT, Araujo AP (2016) Zika virus‐associated neurological disorders: a review. Brain 139(Pt 8):2122–2130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

