An In Vitro Microneutralization Assay for SARS-CoV-2 Serology and Drug Screening
- PMID: 32585083
- PMCID: PMC7361222
- DOI: 10.1002/cpmc.108
An In Vitro Microneutralization Assay for SARS-CoV-2 Serology and Drug Screening
Abstract
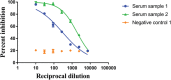
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in the city of Wuhan, Hubei Province, China, in late 2019. Since then, the virus has spread globally and caused a pandemic. Assays that can measure the antiviral activity of antibodies or antiviral compounds are needed for SARS-CoV-2 vaccine and drug development. Here, we describe in detail a microneutralization assay, which can be used to assess in a quantitative manner if antibodies or drugs can block entry and/or replication of SARS-CoV-2 in vitro. © 2020 Wiley Periodicals LLC. Basic Protocol 1: Microneutralization assay to test inhibition of virus by antibodies (purified antibodies or serum/plasma) Basic Protocol 2: Screening of anti-SARS-CoV-2 compounds in vitro Support Protocol: SARS-CoV-2 propagation.
Keywords: COVID-19; COVID19; SARS-CoV-2; antivirals; medium-throughput screening; microneutralization; neutralization.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
Mount Sinai has licensed serological assays to commercial entities and has filed for patent protection for serological assays.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

