Comprehensive Endogenous Tagging of Basement Membrane Components Reveals Dynamic Movement within the Matrix Scaffolding
- PMID: 32585132
- PMCID: PMC7394237
- DOI: 10.1016/j.devcel.2020.05.022
Comprehensive Endogenous Tagging of Basement Membrane Components Reveals Dynamic Movement within the Matrix Scaffolding
Abstract
Basement membranes (BMs) are supramolecular matrices built on laminin and type IV collagen networks that provide structural and signaling support to tissues. BM complexity, however, has hindered an understanding of its formation, dynamics, and regulation. Using genome editing, we tagged 29 BM matrix components and receptors in C. elegans with mNeonGreen. Here, we report a common template that initiates BM formation, which rapidly diversifies during tissue differentiation. Through photobleaching studies, we show that BMs are not static-surprisingly, many matrix proteins move within the laminin and collagen scaffoldings. Finally, quantitative imaging, conditional knockdown, and optical highlighting indicate that papilin, a poorly studied glycoprotein, is the most abundant component in the gonadal BM, where it facilitates type IV collagen removal during BM expansion and tissue growth. Together, this work introduces methods for holistic investigation of BM regulation and reveals that BMs are highly dynamic and capable of rapid change to support tissues.
Keywords: C. elegans; basement membrane; basement membrane dynamics; endogenous tagging; extracellular matrix; laminin; organ growth; papilin; type IV collagen.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



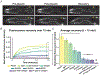


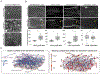
Comment in
-
Mobilizing the Matrix for Organ Morphogenesis.Dev Cell. 2020 Jul 6;54(1):1-2. doi: 10.1016/j.devcel.2020.06.014. Dev Cell. 2020. PMID: 32634396
Similar articles
-
SPARC Promotes Cell Invasion In Vivo by Decreasing Type IV Collagen Levels in the Basement Membrane.PLoS Genet. 2016 Feb 29;12(2):e1005905. doi: 10.1371/journal.pgen.1005905. eCollection 2016 Feb. PLoS Genet. 2016. PMID: 26926673 Free PMC article.
-
Basement Membranes in the Worm: A Dynamic Scaffolding that Instructs Cellular Behaviors and Shapes Tissues.Curr Top Membr. 2015;76:337-71. doi: 10.1016/bs.ctm.2015.08.001. Epub 2015 Sep 12. Curr Top Membr. 2015. PMID: 26610919 Free PMC article. Review.
-
Genetic Suppression of Basement Membrane Defects in Caenorhabditis elegans by Gain of Function in Extracellular Matrix and Cell-Matrix Attachment Genes.Genetics. 2018 Apr;208(4):1499-1512. doi: 10.1534/genetics.118.300731. Epub 2018 Feb 12. Genetics. 2018. PMID: 29440357 Free PMC article.
-
α-Integrins dictate distinct modes of type IV collagen recruitment to basement membranes.J Cell Biol. 2019 Sep 2;218(9):3098-3116. doi: 10.1083/jcb.201903124. Epub 2019 Aug 6. J Cell Biol. 2019. PMID: 31387941 Free PMC article.
-
Control of the basement membrane and cell migration by ADAMTS proteinases: Lessons from C. elegans genetics.Matrix Biol. 2015 May-Jul;44-46:64-9. doi: 10.1016/j.matbio.2015.01.001. Epub 2015 Jan 14. Matrix Biol. 2015. PMID: 25595837 Review.
Cited by
-
Complexities of the glomerular basement membrane.Nat Rev Nephrol. 2021 Feb;17(2):112-127. doi: 10.1038/s41581-020-0329-y. Epub 2020 Aug 24. Nat Rev Nephrol. 2021. PMID: 32839582 Review.
-
Structural and physiological functions of Caenorhabditis elegans epidermis.Heliyon. 2024 Sep 28;10(19):e38680. doi: 10.1016/j.heliyon.2024.e38680. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39397934 Free PMC article. Review.
-
Niche cells regulate primordial germ cell quiescence in response to basement membrane signaling.Development. 2023 Aug 15;150(16):dev201640. doi: 10.1242/dev.201640. Epub 2023 Aug 17. Development. 2023. PMID: 37497562 Free PMC article.
-
Basement membranes in obstructive pulmonary diseases.Matrix Biol Plus. 2021 Nov 12;12:100092. doi: 10.1016/j.mbplus.2021.100092. eCollection 2021 Dec. Matrix Biol Plus. 2021. PMID: 34877523 Free PMC article. Review.
-
Signaling circuits and the apical extracellular matrix in aging: connections identified in the nematode Caenorhabditis elegans.Am J Physiol Cell Physiol. 2023 Nov 1;325(5):C1201-C1211. doi: 10.1152/ajpcell.00195.2023. Epub 2023 Sep 18. Am J Physiol Cell Physiol. 2023. PMID: 37721005 Free PMC article. Review.
References
-
- Candiello J, Cole GJ, and Halfter W (2010). Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol 29, 402–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

