Bone morphogenic proteins in iron homeostasis
- PMID: 32585319
- PMCID: PMC7453787
- DOI: 10.1016/j.bone.2020.115495
Bone morphogenic proteins in iron homeostasis
Abstract
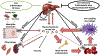
The bone morphogenetic protein (BMP)-SMAD signaling pathway plays a central role in regulating hepcidin, which is the master hormone governing systemic iron homeostasis. Hepcidin is produced by the liver and acts on the iron exporter ferroportin to control iron absorption from the diet and iron release from body stores, thereby providing adequate iron for red blood cell production, while limiting the toxic effects of excess iron. BMP6 and BMP2 ligands produced by liver endothelial cells bind to BMP receptors and the coreceptor hemojuvelin (HJV) on hepatocytes to activate SMAD1/5/8 signaling, which directly upregulates hepcidin transcription. Most major signals that influence hepcidin production, including iron, erythropoietic drive, and inflammation, intersect with the BMP-SMAD pathway to regulate hepcidin transcription. Mutation or inactivation of BMP ligands, BMP receptors, HJV, SMADs or other proteins that modulate the BMP-SMAD pathway result in hepcidin dysregulation, leading to iron-related disorders, such as hemochromatosis and iron refractory iron deficiency anemia. Pharmacologic modulators of the BMP-SMAD pathway have shown efficacy in pre-clinical models to regulate hepcidin expression and treat iron-related disorders. This review will discuss recent insights into the role of the BMP-SMAD pathway in regulating hepcidin to control systemic iron homeostasis.
Keywords: Anemia; Bone morphogenetic protein; Hemochromatosis; Hemojuvelin; Hepcidin; Iron; SMAD.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest JLB has ownership interest in Ferrumax Pharmaceuticals. All other authors declare no conflict of interest.
Figures

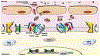

Similar articles
-
Hepcidin-mediated hypoferremic response to acute inflammation requires a threshold of Bmp6/Hjv/Smad signaling.Blood. 2018 Oct 25;132(17):1829-1841. doi: 10.1182/blood-2018-03-841197. Epub 2018 Sep 13. Blood. 2018. PMID: 30213871
-
Down-regulation of Bmp/Smad signaling by Tmprss6 is required for maintenance of systemic iron homeostasis.Blood. 2010 May 6;115(18):3817-26. doi: 10.1182/blood-2009-05-224808. Epub 2010 Mar 3. Blood. 2010. PMID: 20200349 Free PMC article.
-
Hepcidin and the BMP-SMAD pathway: An unexpected liaison.Vitam Horm. 2019;110:71-99. doi: 10.1016/bs.vh.2019.01.004. Epub 2019 Feb 10. Vitam Horm. 2019. PMID: 30798817 Review.
-
Coordination of iron homeostasis by bone morphogenetic proteins: Current understanding and unanswered questions.Dev Dyn. 2022 Jan;251(1):26-46. doi: 10.1002/dvdy.372. Epub 2021 May 25. Dev Dyn. 2022. PMID: 33993583 Free PMC article.
-
Bone morphogenetic proteins as regulators of iron metabolism.Annu Rev Nutr. 2014;34:77-94. doi: 10.1146/annurev-nutr-071813-105646. Epub 2014 Jun 6. Annu Rev Nutr. 2014. PMID: 24995692 Free PMC article. Review.
Cited by
-
Vitamin E Induces Liver Iron Depletion and Alters Iron Regulation in Mice.J Nutr. 2023 Jul;153(7):1866-1876. doi: 10.1016/j.tjnut.2023.04.018. Epub 2023 Apr 29. J Nutr. 2023. PMID: 37127137 Free PMC article.
-
Menstrual cycle affects iron homeostasis and hepcidin following interval running exercise in endurance-trained women.Eur J Appl Physiol. 2022 Dec;122(12):2683-2694. doi: 10.1007/s00421-022-05048-5. Epub 2022 Sep 21. Eur J Appl Physiol. 2022. PMID: 36129579 Free PMC article.
-
The GPR30-Mediated BMP-6/HEP/FPN Signaling Pathway Inhibits Ferroptosis in Bone Marrow Mesenchymal Stem Cells to Alleviate Osteoporosis.Int J Mol Sci. 2025 Feb 26;26(5):2027. doi: 10.3390/ijms26052027. Int J Mol Sci. 2025. PMID: 40076648 Free PMC article.
-
Revisiting hemochromatosis: genetic vs. phenotypic manifestations.Ann Transl Med. 2021 Apr;9(8):731. doi: 10.21037/atm-20-5512. Ann Transl Med. 2021. PMID: 33987429 Free PMC article. Review.
-
UBA6 and NDFIP1 regulate the degradation of ferroportin.Haematologica. 2022 Feb 1;107(2):478-488. doi: 10.3324/haematol.2021.278530. Haematologica. 2022. PMID: 34320783 Free PMC article.
References
-
- Gammella E, Buratti P, Cairo G, Recalcati S, The transferrin receptor: the cellular iron gate, Metallomics 9(10) (2017) 1367–1375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

