Gonadotrophins or clomiphene citrate in women with normogonadotropic anovulation and CC failure: does the endometrium matter?
- PMID: 32585686
- PMCID: PMC7316496
- DOI: 10.1093/humrep/deaa052
Gonadotrophins or clomiphene citrate in women with normogonadotropic anovulation and CC failure: does the endometrium matter?
Abstract
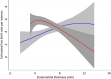
Study question: Is endometrial thickness (EMT) a biomarker to select between women who should switch to gonadotropins and those who could continue clomiphene citrate (CC) after six failed ovulatory cycles?
Summary answer: Using a cut-off of 7 mm for EMT, we can distinguish between women who are better off switching to gonadotropins and those who could continue CC after six earlier failed ovulatory CC cycles.
What is already known: For women with normogonadotropic anovulation, CC has been a long-standing first-line treatment in conjunction with intercourse or intrauterine insemination (IUI). We recently showed that a switch to gonadotropins increases the chance of live birth by 11% in these women over continued treatment with CC after six failed ovulatory cycles, at a cost of €15 258 per additional live birth. It is unclear whether EMT can be used to identify women who can continue on CC with similar live birth rates without the extra costs of gonadotropins.
Study design, size, duration: Between 8 December 2008 and 16 December 2015, 666 women with CC failure were randomly assigned to receive an additional six cycles with a change to gonadotropins (n = 331) or an additional six cycles continuing with CC (n = 335), both in conjunction with intercourse or IUI. The primary outcome was conception leading to live birth within 8 months after randomisation. EMT was measured mid-cycle before randomisation during their sixth ovulatory CC cycle. The EMT was available in 380 women, of whom 190 were allocated to gonadotropins and 190 were allocated to CC.
Participants/materials, setting, methods: EMT was determined in the sixth CC cycle prior to randomisation. We tested for interaction of EMT with the treatment effect using logistic regression. We performed a spline analysis to evaluate the association of EMT with chance to pregnancy leading to a live birth in the next cycles and to determine the best cut-off point. On the basis of the resulting cut-off point, we calculated the relative risk and 95% CI of live birth for gonadotropins versus CC at EMT values below and above this cut-off point. Finally, we calculated incremental cost-effectiveness ratios (ICER).
Main results and the role of chance: Mid-cycle EMT in the sixth cycle interacted with treatment effect (P < 0.01). Spline analyses showed a cut-off point of 7 mm. There were 162 women (45%) who had an EMT ≤ 7 mm in the sixth ovulatory cycle and 218 women (55%) who had an EMT > 7 mm. Among the women with EMT ≤ 7 mm, gonadotropins resulted in a live birth in 44 of 79 women (56%), while CC resulted in a live birth in 28 of 83 women (34%) (RR 1.57, 95% CI 1.13-2.19). Per additional live birth with gonadotropins, the ICER was €9709 (95% CI: €5117 to €25 302). Among the women with EMT > 7 mm, gonadotropins resulted in a live birth in 53 of 111 women (48%) while CC resulted in a live birth in 52 of 107 women (49%) (RR 0.98, 95% CI 0.75-1.29).
Limitations, reasons for caution: This was a post hoc analysis of a randomised controlled trial (RCT) and therefore mid-cycle EMT measurements before randomisation during their sixth ovulatory CC cycle were not available for all included women.
Wider implications of the findings: In women with six failed ovulatory cycles on CC and an EMT ≤ 7 mm in the sixth cycle, we advise switching to gonadotropins, since it improves live birth rate over continuing treatment with CC at an extra cost of €9709 to achieve one additional live birth. If the EMT > 7 mm, we advise to continue treatment with CC, since live birth rates are similar to those with gonadotropins, without the extra costs.
Study funding/competing interest(s): The original MOVIN trial received funding from the Dutch Organization for Health Research and Development (ZonMw number: 80-82310-97-12067). C.B.L.A. reports unrestricted grant support from Merck and Ferring. B.W.M. is supported by a NHMRC Practitioner Fellowship (GNT1082548) and reports consultancy for Merck, ObsEva, IGENOMIX and Guerbet. All other authors have nothing to declare.
Trial registration number: Netherlands Trial Register, number NTR1449.
Keywords: PCOS; clomiphene citrate; endometrial thickness; gonadotropins; ovulation induction.
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology.
Figures




References
-
- Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, Stener-Victorin E, Fauser BC, Norman RJ, Teede H. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update 2016;22:687–708. - PubMed
-
- Bordewijk EM, Weiss NS, Nahuis MJ, Bayram N, Hooff MHA, Boks DES, Perquin DAM, Janssen CAH, Golde RJT, Lambalk CB et al. . Gonadotrophins versus clomiphene citrate with or without IUI in women with normogonadotropic anovulation and clomiphene failure: a cost-effectiveness analysis. Hum Reprod 2019;34:276–284. - PubMed
-
- Costello MF, Misso ML, Balen A, Boyle J, Devoto L, Garad RM, Hart R, Johnson L, Jordan C, Legro RS et al. . Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: assessment and treatment of infertility. Human Reprod Open 2019b;2019:1–24. - PMC - PubMed
-
- Gadalla MA, Huang S, Wang R, Norman RJ, Abdullah SA, El Saman AM, Ismail AM, Wely M, Mol BWJ. Effect of clomiphene citrate on endometrial thickness, ovulation, pregnancy and live birth in anovulatory women: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;51:64–76. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

