Structural Insights into RNA Dimerization: Motifs, Interfaces and Functions
- PMID: 32585844
- PMCID: PMC7357161
- DOI: 10.3390/molecules25122881
Structural Insights into RNA Dimerization: Motifs, Interfaces and Functions
Abstract
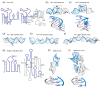
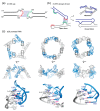
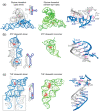
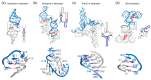
In comparison with the pervasive use of protein dimers and multimers in all domains of life, functional RNA oligomers have so far rarely been observed in nature. Their diminished occurrence contrasts starkly with the robust intrinsic potential of RNA to multimerize through long-range base-pairing ("kissing") interactions, self-annealing of palindromic or complementary sequences, and stable tertiary contact motifs, such as the GNRA tetraloop-receptors. To explore the general mechanics of RNA dimerization, we performed a meta-analysis of a collection of exemplary RNA homodimer structures consisting of viral genomic elements, ribozymes, riboswitches, etc., encompassing both functional and fortuitous dimers. Globally, we found that domain-swapped dimers and antiparallel, head-to-tail arrangements are predominant architectural themes. Locally, we observed that the same structural motifs, interfaces and forces that enable tertiary RNA folding also drive their higher-order assemblies. These feature prominently long-range kissing loops, pseudoknots, reciprocal base intercalations and A-minor interactions. We postulate that the scarcity of functional RNA multimers and limited diversity in multimerization motifs may reflect evolutionary constraints imposed by host antiviral immune surveillance and stress sensing. A deepening mechanistic understanding of RNA multimerization is expected to facilitate investigations into RNA and RNP assemblies, condensates, and granules and enable their potential therapeutical targeting.
Keywords: RNA; dimerization; domain swapping; folding; intermolecular interaction; riboswitches; ribozymes; structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

