In Vitro and In Vivo Assessment of the Efficacy of Bromoageliferin, an Alkaloid Isolated from the Sponge Agelas dilatata, against Pseudomonas aeruginosa
- PMID: 32585891
- PMCID: PMC7345159
- DOI: 10.3390/md18060326
In Vitro and In Vivo Assessment of the Efficacy of Bromoageliferin, an Alkaloid Isolated from the Sponge Agelas dilatata, against Pseudomonas aeruginosa
Abstract
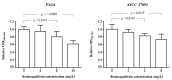
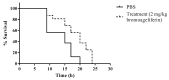
The pyrrole-imidazoles, a group of alkaloids commonly found in marine sponges belonging to the genus Agelas, display a wide range of biological activities. Herein, we report the first chemical study of the secondary metabolites of the sponge A. dilatata from the coastal area of the Yucatan Peninsula (Mexico). In this study, we isolated eight known alkaloids from an organic extract of the sponge. We used NMR and MS analysis and comparison with existing databases to characterize the alkaloids: ageliferin (1), bromoageliferin (2), dibromoageliferin (3), sceptrin (4), nakamuric acid (5), 4-bromo-1H-pyrrole-2-carboxylic acid (6), 4,5-dibromopyrrole-2-carboxylic acid (7) and 3,7-dimethylisoguanine (8). We also evaluated, for the first time, the activity of these alkaloids against the most problematic multidrug-resistant (MDR) pathogens, i.e., the Gram-negative bacteria Pseudomonas aeruginosa, Klebsiella pneumoniae and Acinetobacter baumannii. Bromoageliferin (2) displayed significant activity against P. aeruginosa. Comparison of the antibacterial activity of ageliferins 1-3 (of similar structure) against P. aeruginosa revealed some relationship between structure and activity. Furthermore, in in vitro assays, 2 inhibited growth and biofilm production in clinical strains of P. aeruginosa. Moreover, 2 increased the survival time in an in vivo Galleria mellonella model of infection. The findings confirm bromoageliferin (2) as a potential lead for designing new antibacterial drugs.
Keywords: Agelas dilatata; Galleria mellonella; Pseudomonas aeruginosa; Yucatan Peninsula; antibacterial; biofilm inhibition; pyrrole-imidazole alkaloids; structure-activity relationships.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Feature-Based Molecular Networking Discovery of Bromopyrrole Alkaloids from the Marine Sponge Agelas dispar.J Nat Prod. 2022 May 27;85(5):1340-1350. doi: 10.1021/acs.jnatprod.2c00094. Epub 2022 Apr 15. J Nat Prod. 2022. PMID: 35427139 Free PMC article. Review.
-
Antimicrobial Diterpene Alkaloids from an Agelas citrina Sponge Collected in the Yucatán Peninsula.Mar Drugs. 2022 Apr 28;20(5):298. doi: 10.3390/md20050298. Mar Drugs. 2022. PMID: 35621949 Free PMC article.
-
Marine Organisms from the Yucatan Peninsula (Mexico) as a Potential Natural Source of Antibacterial Compounds.Mar Drugs. 2020 Jul 18;18(7):369. doi: 10.3390/md18070369. Mar Drugs. 2020. PMID: 32708418 Free PMC article.
-
New bromopyrrole alkaloids from the Indopacific sponge Agelas nakamurai.J Nat Prod. 1999 Sep;62(9):1295-7. doi: 10.1021/np990071f. J Nat Prod. 1999. PMID: 10514317
-
Bromopyrrole Alkaloids from Okinawan Marine Sponges Agelas spp.Chem Pharm Bull (Tokyo). 2016;64(7):691-4. doi: 10.1248/cpb.c16-00245. Chem Pharm Bull (Tokyo). 2016. PMID: 27373625 Review.
Cited by
-
Antimicrobial and cytotoxic effects of marine sponge extracts Agelas clathrodes, Desmapsamma anchorata and Verongula rigida from a Caribbean Island.PeerJ. 2022 Sep 23;10:e13955. doi: 10.7717/peerj.13955. eCollection 2022. PeerJ. 2022. PMID: 36172499 Free PMC article.
-
Recent Advances in Marine-Derived Compounds as Potent Antibacterial and Antifungal Agents: A Comprehensive Review.Mar Drugs. 2024 Jul 29;22(8):348. doi: 10.3390/md22080348. Mar Drugs. 2024. PMID: 39195465 Free PMC article. Review.
-
Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review.Antibiotics (Basel). 2021 Mar 19;10(3):318. doi: 10.3390/antibiotics10030318. Antibiotics (Basel). 2021. PMID: 33808601 Free PMC article. Review.
-
Discovery of Marine Natural Products as Promising Antibiotics against Pseudomonas aeruginosa.Mar Drugs. 2022 Mar 4;20(3):192. doi: 10.3390/md20030192. Mar Drugs. 2022. PMID: 35323491 Free PMC article. Review.
-
β-Lactams from the Ocean.Mar Drugs. 2023 Jan 25;21(2):86. doi: 10.3390/md21020086. Mar Drugs. 2023. PMID: 36827127 Free PMC article. Review.
References
-
- World Health Organization (WHO) Antimicrobial Resistance-Global Report on Surveillance. [(accessed on 23 May 2014)];2014 Available online: http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

