Survival Outcomes of Patients with Pathologically Proven Positive Lymph Nodes at Time of Radical Cystectomy with or without Neoadjuvant Chemotherapy
- PMID: 32585894
- PMCID: PMC7356776
- DOI: 10.3390/jcm9061962
Survival Outcomes of Patients with Pathologically Proven Positive Lymph Nodes at Time of Radical Cystectomy with or without Neoadjuvant Chemotherapy
Abstract
Background: To compare overall survival (OS) outcomes in pN1-3 disease at the time of radical cystectomy (RC) for muscle invasive bladder according to the neoadjuvant chemotherapy (NAC) status.
Materials and methods: This multicenter study included 450 consecutive patients undergoing RC for muscle-invasive urothelial bladder cancer with pN1-3 pM0 disease from 2010 to 2019. NAC consisted in platinum-based chemotherapy. The primary endpoint was the comparison between NAC and non-NAC in terms of death from any cause. OS was assessed using the Kaplan-Meier method and multivariate Cox proportional hazards regression was used to estimate adjusted hazard ratios.
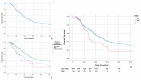
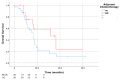
Results: Median age was 69 years. Patients receiving NAC were younger (p = 0.051), and more likely had downstaging to non-muscle invasive disease (10.7% versus 4.3%, p = 0.042). Median OS was 26.6 months. NAC patients had poorer OS compared with those who did receive NAC (Hazard ratio (HR) 1.6; p = 0.019). The persistence of muscle-invasive bladder in RC specimens was also significantly associated with OS (HR 2.40). In the NAC cohort, the two factors independently correlated with OS were the number of positive lymph nodes (p = 0.013) and adjuvant chemotherapy (AC) (HR 0.31; p = 0.015).
Conclusions: Persistent nodal disease in RC specimens after NAC was associated with poor prognosis and lower OS rates compared with pN1-3 disease after upfront RC. In this sub-group of NAC patients, AC was independently associated with better OS.
Keywords: adjuvant; bladder cancer; chemotherapy; neoadjuvant; nodal disease; pN1; radical cystectomy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Griffiths G., Hall R., Sylvester R., Raghavan D., Parmar M.K., Club Urologico Espanol de Tratamiento Oncologico Group International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Longterm results of the BA06 30894 trial. J. Clin. Oncol. 2011;29:2171. - PMC - PubMed
-
- Grossman H.B., Natale R.B., Tangen C.M., Speights V.O., Vogelzang N.J., Trump D.L., White R.W.D., Sarosdy M.F., Wood D.P., Jr., Raghavan D., et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. - DOI - PubMed
-
- Vale C.L., Advanced Bladder Cancer (ABC) Meta-analysis Collaboration Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta analysis collaboration. Eur. Urol. 2005;48:202–206. doi: 10.1016/j.eururo.2005.04.006. - DOI - PubMed
-
- Zargar-Shoshtari K., Zargar H., Lotan Y., Shah J.B., van Rhijn B.W., Daneshmand S., Spiess P.E., Black P.C., Fairey L.S.M.C.A.S., Fairey A.S., et al. A Multi-Institutional Analysis of Outcomes of Patients with Clinically Node Positive Urothelial Bladder Cancer Treated with Induction Chemotherapy and Radical Cystectomy. J. Urol. 2016;195:53–59. doi: 10.1016/j.juro.2015.07.085. - DOI - PubMed
-
- Mertens L.S., Meijer R.P., Meinhardt W., Van Der Poel H.G., Bex A., Kerst J.M., Van Der Heijden M.S., Bergman A.M., Horenblas S., Van Rhijn B.W.G. Occult lymph node metastases in patients with carcinoma invading bladder muscle: Incidence after neoadjuvant chemotherapy and cystectomy vs. after cystectomy alone. BJU Int. 2014;114:67–74. doi: 10.1111/bju.12447. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

