Combined Therapy with Anti-PD1 and BRAF and/or MEK Inhibitor for Advanced Melanoma: A Multicenter Cohort Study
- PMID: 32585901
- PMCID: PMC7352575
- DOI: 10.3390/cancers12061666
Combined Therapy with Anti-PD1 and BRAF and/or MEK Inhibitor for Advanced Melanoma: A Multicenter Cohort Study
Abstract
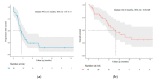
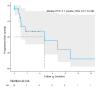
Despite significant progress in melanoma survival, therapeutic options are still needed in case of progression under immune checkpoint inhibitors (ICI), and resistance to targeted therapies (TT) in BRAF-mutated melanomas. This study aimed to assess the safety of combined ICI and TT as a rescue line in real-life clinical practice. We conducted a study within the prospective French multicentric MelBase cohort, including patients treated with a combination of anti-PD1 (pembrolizumab/nivolumab) and BRAF inhibitor (BRAFi: dabrafenib/vemurafenib) and/or MEK inhibitors (MEKi: trametinib/cobimetinib) for BRAF mutated or wild-type advanced melanoma. Fifty-nine patients were included: 30% received the triple combination, 34% an anti-PD1 and BRAFi, and 36% an anti-PD1 and MEKi. Grade 3-4 adverse events occurred in 12% of patients. Permanent discontinuation or dose reduction of one of the treatments for toxicity was reported in 14% and 7% of patients, respectively. In the BRAF wild-type subgroup, treatment with MEKi and anti-PD1 induced a tumor control rate of 83% and median progression-free survival of 7.1 months. The combination of anti-PD1 and BRAFi and/or MEKi was a safe rescue line for advanced melanoma patients previously treated with ICI/TT. The benefit of these combinations, specifically anti-PD1 and MEKi in BRAF wild-type melanoma patients, needs to be prospectively studied.
Keywords: BRAF inhibitor; MEK inhibitor; anti-PD1; melanoma; targeted therapy.
Conflict of interest statement
C.L.: reports grants and personal fees from Bristol-Myers Squibb, personal fees from MSD, personal fees from Novartis, personal fees from Amgen, grants and personal fees from Roche, personal fees from Avantis Medical Systems, personal fees from Pierre Fabre, personal fees from Pfizer, personal fees from Incyte, personal fees from Merck Serono, personal fees from Sanofi, outside the submitted work. J.D.: reports travel accommodations from Pierre Fabre and Roche, outside the submitted work. L.M.: reports personal fees from Bristol-Myers Squibb, personal fees from Roche, personal fees from MSD, personal fees from Novartis, personal fees from Merck, outside the submitted work. J.P.A.: reports personal fees from Bristol-Myers-Squibb, grants from BMS—Novartis—MSD, during the conduct of the study. B.D., C.D., E.M., F.A., F.B.P., M.B., M.B.B., M.T.L., O.D., S.H., W.L.: declare no conflict of interest.
Figures
References
-
- Long G.V., Stroyakovskiy D., Gogas H., Levchenko E., de Braud F., Larkin J., Garbe C., Jouary T., Hauschild A., Grob J.-J., et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444–451. doi: 10.1016/S0140-6736(15)60898-4. - DOI - PubMed
-
- Long G.V., Flaherty K.T., Stroyakovskiy D., Gogas H., Levchenko E., de Braud F., Larkin J., Garbe C., Jouary T., Hauschild A., et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Annal. Oncol. 2017;28:1631–1639. doi: 10.1093/annonc/mdx176. - DOI - PMC - PubMed
-
- Ascierto P.A., McArthur G.A., Dréno B., Atkinson V., Liszkay G., Di Giacomo A.M., Mandalà M., Demidov L., Stroyakovskiy D., Thomas L., et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248–1260. doi: 10.1016/S1470-2045(16)30122-X. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

