Glucose Metabolism and Oxidative Stress in Hepatocellular Carcinoma: Role and Possible Implications in Novel Therapeutic Strategies
- PMID: 32585931
- PMCID: PMC7352479
- DOI: 10.3390/cancers12061668
Glucose Metabolism and Oxidative Stress in Hepatocellular Carcinoma: Role and Possible Implications in Novel Therapeutic Strategies
Abstract
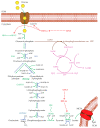
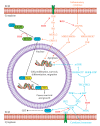
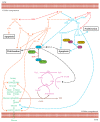
Hepatocellular carcinoma (HCC) metabolism is redirected to glycolysis to enhance the production of metabolic compounds employed by cancer cells to produce proteins, lipids, and nucleotides in order to maintain a high proliferative rate. This mechanism drives towards uncontrolled growth and causes a further increase in reactive oxygen species (ROS), which could lead to cell death. HCC overcomes the problem generated by ROS increase by increasing the antioxidant machinery, in which key mechanisms involve glutathione, nuclear factor erythroid 2-related factor 2 (Nrf2), and hypoxia-inducible transcription factor (HIF-1α). These mechanisms could represent optimal targets for innovative therapies. The tumor microenvironment (TME) exerts a key role in HCC pathogenesis and progression. Various metabolic machineries modulate the activity of immune cells in the TME. The deregulated metabolic activity of tumor cells could impair antitumor response. Lactic acid-lactate, derived from the anaerobic glycolytic rate of tumor cells, as well as adenosine, derived from the catabolism of ATP, have an immunosuppressive activity. Metabolic reprogramming of the TME via targeted therapies could enhance the treatment efficacy of anti-cancer immunotherapy. This review describes the metabolic pathways mainly involved in the HCC pathogenesis and progression. The potential targets for HCC treatment involved in these pathways are also discussed.
Keywords: HCC; anticancer-immunoresponse; glucose metabolism; oxidative stress; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Tumor microenvironment and metabolic synergy in breast cancers: critical importance of mitochondrial fuels and function.Semin Oncol. 2014 Apr;41(2):195-216. doi: 10.1053/j.seminoncol.2014.03.002. Epub 2014 Mar 5. Semin Oncol. 2014. PMID: 24787293 Review.
-
Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma.Acta Pharm Sin B. 2022 Feb;12(2):558-580. doi: 10.1016/j.apsb.2021.09.019. Epub 2021 Sep 25. Acta Pharm Sin B. 2022. PMID: 35256934 Free PMC article. Review.
-
Lactic acid: a narrative review of a promoter of the liver cancer microenvironment.J Gastrointest Oncol. 2024 Jun 30;15(3):1282-1296. doi: 10.21037/jgo-24-368. Epub 2024 Jun 13. J Gastrointest Oncol. 2024. PMID: 38989406 Free PMC article. Review.
-
MiR-3662 suppresses hepatocellular carcinoma growth through inhibition of HIF-1α-mediated Warburg effect.Cell Death Dis. 2018 May 1;9(5):549. doi: 10.1038/s41419-018-0616-8. Cell Death Dis. 2018. PMID: 29748591 Free PMC article.
-
HBXIP drives metabolic reprogramming in hepatocellular carcinoma cells via METTL3-mediated m6A modification of HIF-1α.J Cell Physiol. 2021 May;236(5):3863-3880. doi: 10.1002/jcp.30128. Epub 2020 Dec 11. J Cell Physiol. 2021. PMID: 33305825
Cited by
-
Serum resistin and the risk for hepatocellular carcinoma in diabetic patients.World J Gastroenterol. 2023 Jul 21;29(27):4271-4288. doi: 10.3748/wjg.v29.i27.4271. World J Gastroenterol. 2023. PMID: 37545641 Free PMC article. Review.
-
New Insights into the Role of miR-29a in Hepatocellular Carcinoma: Implications in Mechanisms and Theragnostics.J Pers Med. 2021 Mar 18;11(3):219. doi: 10.3390/jpm11030219. J Pers Med. 2021. PMID: 33803804 Free PMC article. Review.
-
Cordycepin Inhibits the Growth of Hepatocellular Carcinoma by Regulating the Pathway of Aerobic Glycolysis.Evid Based Complement Alternat Med. 2022 Nov 23;2022:6454482. doi: 10.1155/2022/6454482. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 36467556 Free PMC article.
-
MHIF-MSEA: a novel model of miRNA set enrichment analysis based on multi-source heterogeneous information fusion.Front Genet. 2024 Mar 22;15:1375148. doi: 10.3389/fgene.2024.1375148. eCollection 2024. Front Genet. 2024. PMID: 38586586 Free PMC article.
-
Multi-omics analysis reveals immunosuppression in oesophageal squamous cell carcinoma induced by creatine accumulation and HK3 deficiency.Genome Med. 2025 May 6;17(1):44. doi: 10.1186/s13073-025-01465-1. Genome Med. 2025. PMID: 40329370 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

