Multilineage Differentiation for Formation of Innervated Skeletal Muscle Fibers from Healthy and Diseased Human Pluripotent Stem Cells
- PMID: 32585982
- PMCID: PMC7349825
- DOI: 10.3390/cells9061531
Multilineage Differentiation for Formation of Innervated Skeletal Muscle Fibers from Healthy and Diseased Human Pluripotent Stem Cells
Abstract
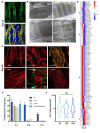
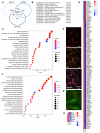
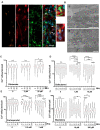
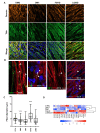
Induced pluripotent stem cells (iPSCs) obtained by reprogramming primary somatic cells have revolutionized the fields of cell biology and disease modeling. However, the number protocols for generating mature muscle fibers with sarcolemmal organization using iPSCs remain limited, and partly mimic the complexity of mature skeletal muscle. Methods: We used a novel combination of small molecules added in a precise sequence for the simultaneous codifferentiation of human iPSCs into skeletal muscle cells and motor neurons. Results: We show that the presence of both cell types reduces the production time for millimeter-long multinucleated muscle fibers with sarcolemmal organization. Muscle fiber contractions are visible in 19-21 days, and can be maintained over long period thanks to the production of innervated multinucleated mature skeletal muscle fibers with autonomous cell regeneration of PAX7-positive cells and extracellular matrix synthesis. The sequential addition of specific molecules recapitulates key steps of human peripheral neurogenesis and myogenesis. Furthermore, this organoid-like culture can be used for functional evaluation and drug screening. Conclusion: Our protocol, which is applicable to hiPSCs from healthy individuals, was validated in Duchenne Muscular Dystrophy, Myotonic Dystrophy, Facio-Scapulo-Humeral Dystrophy and type 2A Limb-Girdle Muscular Dystrophy, opening new paths for the exploration of muscle differentiation, disease modeling and drug discovery.
Keywords: Duchenne Muscular Dystrophy; Facio-Scapulo-Humeral Dystrophy; Limb girdle muscular dystrophy; Myotonic Dystrophy; Z-lines; contraction; differentiation; human induced pluripotent cells; motor neurons; muscular dystrophy; myoblasts; myotubes; satellite cells.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Maffioletti S.M., Gerli M.F., Ragazzi M., Dastidar S., Benedetti S., Loperfido M., VandenDriessche T., Chuah M.K., Tedesco F.S. Efficient derivation and inducible differentiation of expandable skeletal myogenic cells from human es and patient-specific ips cells. Nat. Protoc. 2015;10:941–958. doi: 10.1038/nprot.2015.057. - DOI - PubMed
-
- Pawlowski M., Ortmann D., Bertero A., Tavares J.M., Pedersen R.A., Vallier L., Kotter M.R.N. Inducible and deterministic forward programming of human pluripotent stem cells into neurons, skeletal myocytes, and oligodendrocytes. Stem Cell Rep. 2017;8:803–812. doi: 10.1016/j.stemcr.2017.02.016. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

